Lipid nanosuspension containing clevidipine butyrate, and freeze-drying preparation of lipid nanosuspension
A technology of clevidipine butyrate and nanosuspension, which is applied in the field of preparation of lipid nanosuspension and freeze-dried preparations thereof, can solve the problems of low solubility of clevidipine butyrate, increase safety and the like, and achieve no The effect of drug leakage, increased safety, and low toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Accurately weigh 1.5 g of soybean lecithin and 2.0 g of glycerin, add to 100 ml of distilled water and dissolve to form a dispersion medium (solution A). Add 50 mg of clevidipine butyrate to solution A, and ultrasonically disperse to obtain suspension B. Continue to use a high-speed shearing machine at 20000r / min for 3min and 30000r / min for 2min to obtain colostrum C. Colostrum C was subjected to a high-pressure homogenization method and circulated 10 times at 200 bar, 10 times at 500 bar, and 20 times at 800 bar to prepare clevidipine butyrate lipid nanosuspension.
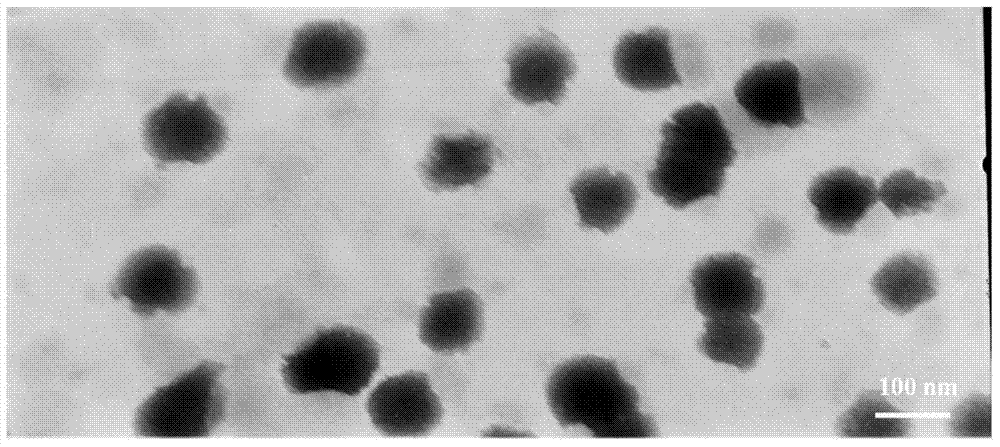
[0040] Add 5.0 g of mannitol to the above-prepared suspension to fully disperse, divide into vials (2ml / bottle), and pre-freeze in a -80°C refrigerator for 24 hours. The pre-frozen sample was placed in a freeze dryer at -40°C and 0.5mbar for 48 hours to freeze-dry for 48 hours to finally obtain a white and loose clevidipine butyrate lipid nanosuspension freeze-dried preparation, such as figure 1 shown. ...
Embodiment 2
[0044] Accurately weigh 1 g of soybean lecithin, add it to 100 ml of distilled water and dissolve it to form a dispersion medium (solution A). Add 50 mg of clevidipine butyrate to solution A, and ultrasonically disperse to obtain suspension B. Continue to use a high-speed shearing machine at 20000r / min for 3min and 30000r / min for 2min to obtain colostrum C. Colostrum C was subjected to a high-pressure homogenization method and circulated 10 times at 200 bar, 10 times at 500 bar, and 20 times at 800 bar to prepare clevidipine butyrate lipid nanosuspension.
[0045] Add 2.0 g of mannitol to the above-prepared suspension to fully disperse, dispense into vials (2 ml / bottle), and pre-freeze in a -80°C refrigerator for 24 hours. The pre-frozen sample was placed in a freeze dryer at -40°C and 0.5 mbar for 48 hours to obtain a white and loose clevidipine butyrate lipid nanosuspension freeze-dried preparation. The lyophilized preparation can be completely reconstituted within 1 min b...
Embodiment 3
[0048] Accurately weigh 1.5 g of soybean lecithin, add it to 100 ml of distilled water and dissolve it to form a dispersion medium (solution A). Add 40mg of clevidipine butyrate to solution A, and ultrasonically disperse evenly to obtain suspension B. Continue to use a high-speed shearing machine at 20000r / min for 3min and 30000r / min for 2min to obtain colostrum C. Colostrum C was subjected to a high-pressure homogenization method and circulated 10 times at 200 bar, 10 times at 500 bar, and 20 times at 800 bar to prepare clevidipine butyrate lipid nanosuspension.
[0049] Add 2.0 g of mannitol to the above-prepared suspension to fully disperse, dispense into vials (2 ml / bottle), and pre-freeze in a -80°C refrigerator for 24 hours. The pre-frozen sample was placed in a freeze dryer at -40°C and 0.5 mbar for 48 hours to obtain a white and loose clevidipine butyrate lipid nanosuspension freeze-dried preparation. The lyophilized preparation can be completely reconstituted within...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Electric potential | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com