Purification method of clevidipine butyrate intermediate
A purification method and carboxylic acid technology, applied in the direction of organic chemistry, etc., can solve the problem of no mention of the removal of by-products, and achieve the effects of easy operation, improving the overall yield and reducing the content of main impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
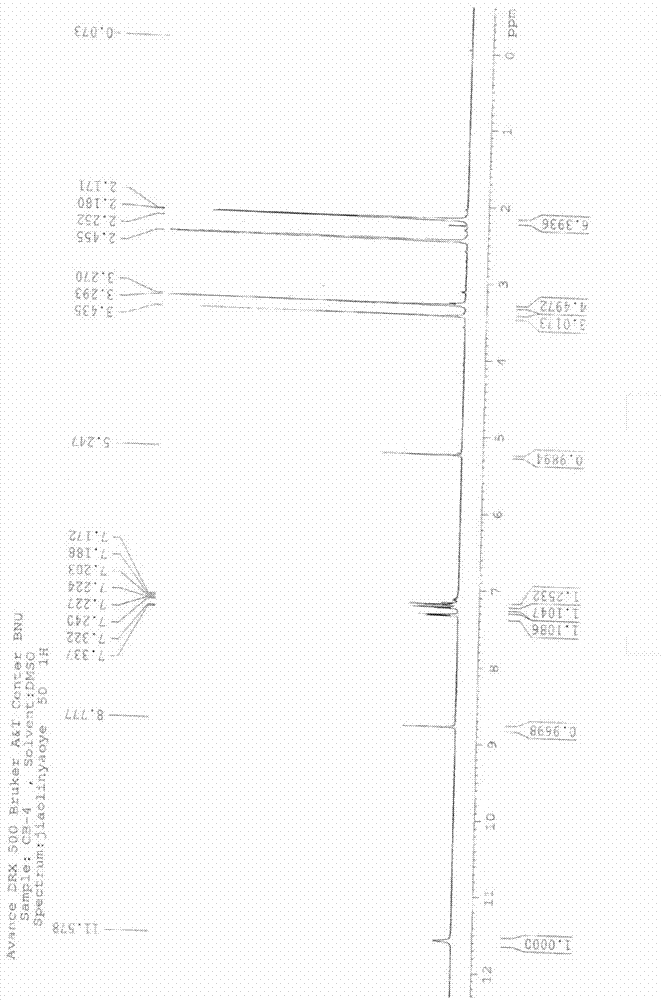
[0033] Example 1: Purification of 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3-carboxylic acid methyl-5-carboxylic acid (II)
[0034]In a three-necked flask, add the crude product of 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3-carboxylic acid methyl-5-carboxylic acid (according to the Literature Chinese journal of pharmaceuticals2011, 42 (7): 484-486 prepared by the same method, in which dicarboxylic acid impurity 5.4%.) 94g (0.264mol), 1050ml isopropanol, cooled with ice water, dropwise added 37.5g 40% (0.375 mol) sodium hydroxide solution, keep the temperature below 20°C, after the addition, stir overnight at room temperature, filter, dissolve the obtained solid in 3L water, decolorize with activated carbon, filter, neutralize the filtrate with hydrochloric acid to pH 5-6, filter, wash with water, and dry , to obtain 84.5 g light yellow solid, yield 90%. HPLC: (99.3%, dicarboxylic acid impurity 0.41%). 1 H NMR (DMSO-d6, ppm): δ=11.57 (s, 1H), 8.7...
Embodiment 2
[0035] Embodiment 2: the synthesis of clevidipine butyrate (Ⅰ)
[0036] In the three-necked flask, add 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3-carboxylic acid methyl-5-carboxylate purified in Example 1 Acid 35.6g (0.1mol), potassium bicarbonate 20g (0.2mol), DMF500ml, add 20.5g (0.15mol) of chloromethyl n-butyrate under stirring, then react at 60°C for 12 hours, cool to room temperature, add to 3L in water, with CH 2 Cl 2 (1L×3) extraction, dried over anhydrous sodium sulfate, concentrated to dryness under reduced pressure, and refined with isopropanol to obtain 40.5 g of white solid with a yield of 89%.
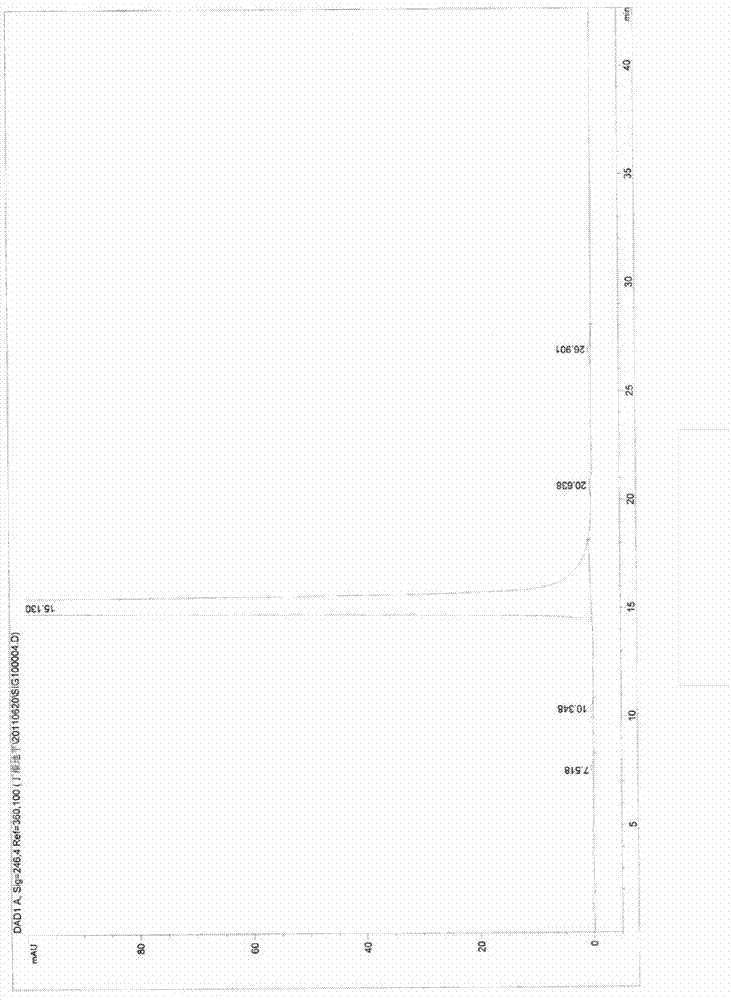
[0037] m.p.: 136.5°C to 138.2°C. HPLC: 99.7%, the main impurity (ie the largest impurity) 0.18%, see figure 2 .
[0038] 1 H NMR (CDCl 3 , ppm): δ=7.01~7.27(m,3H), 6.29(s,1H), 5.73(d,1H),
[0039] 5.68(d,1H), 5.39(s,1H), 3.48(s,1H), 2.19~2.25(m,8H), 1.55(m,2H), 0.83(t,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com