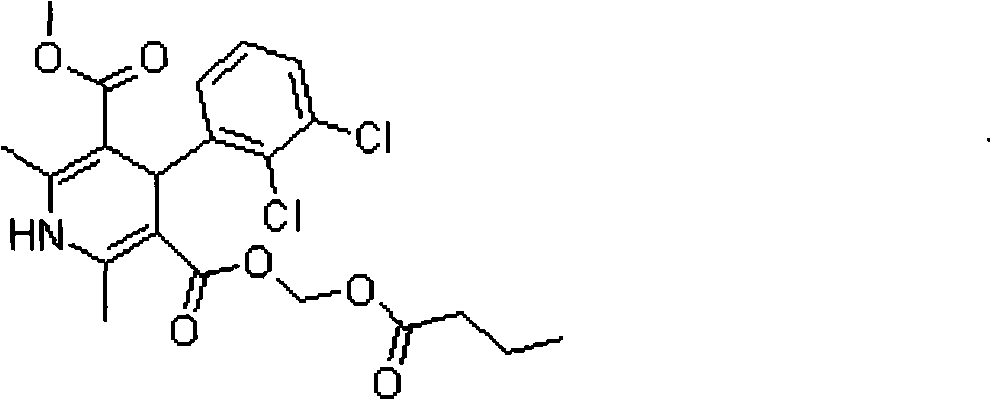
Clevidipine butyrate freeze-dried emulsion
A technology of clevidipine butyrate and freeze-dried emulsion, which is applied in the field of clevidipine butyrate freeze-dried emulsion, can solve the problems of particle aggregation increase, harm, fat emulsion instability, etc., and achieve low toxic and side effects, high curative effect, good stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
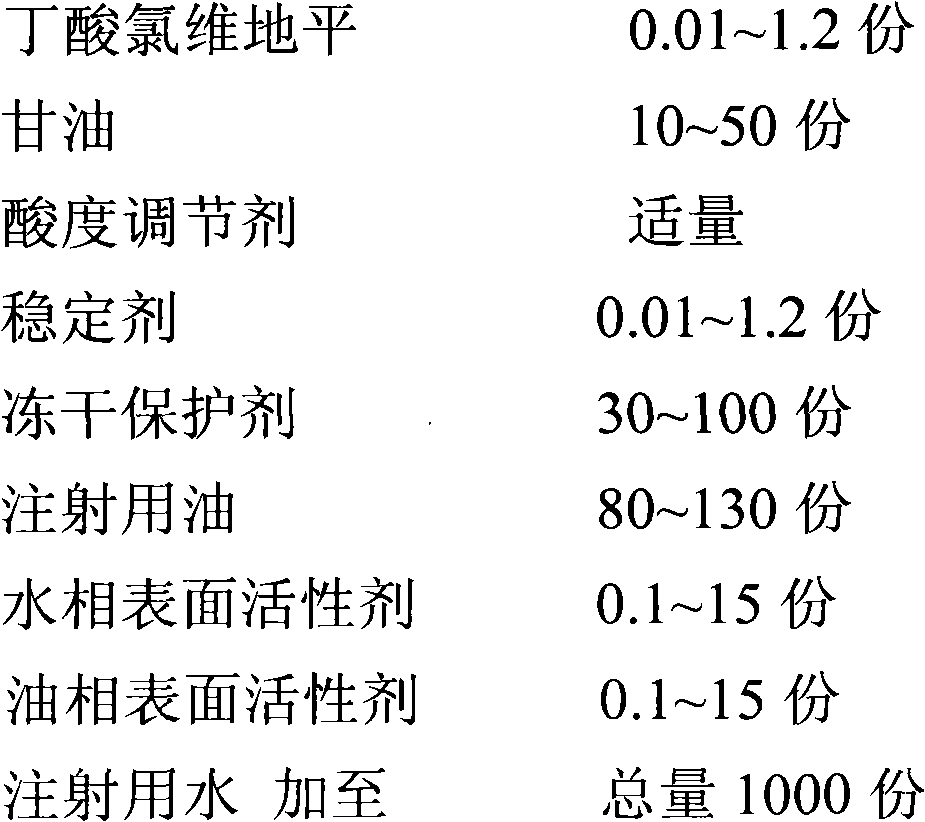
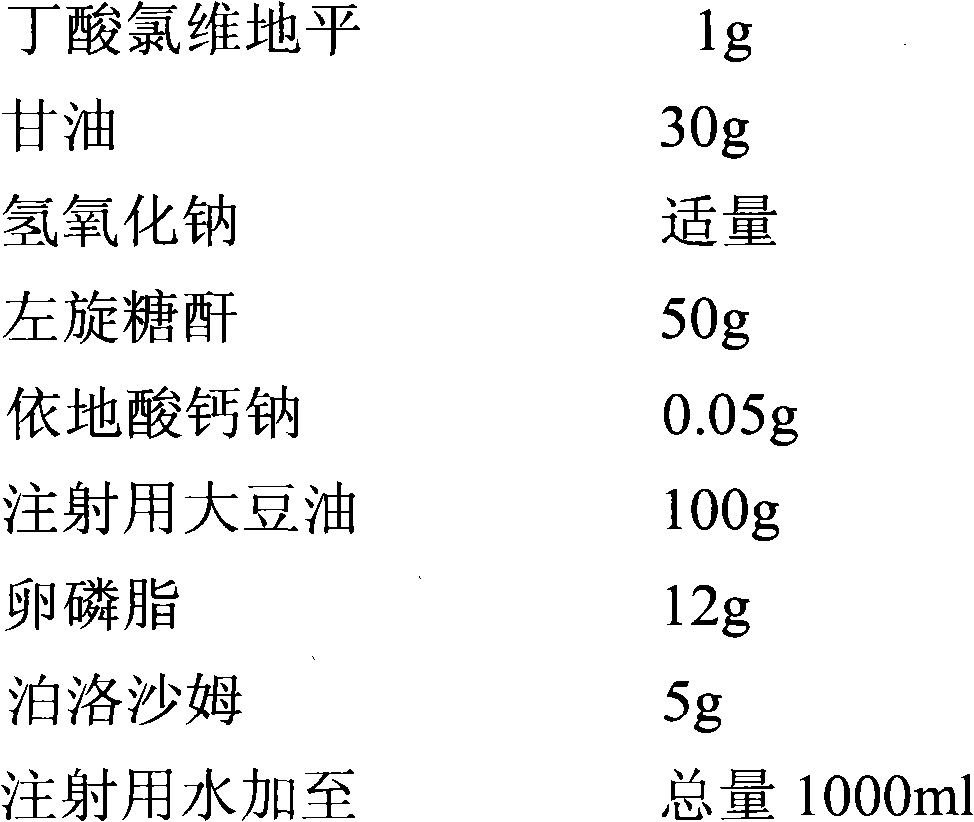
[0036] prescription:
[0037]
[0038] Preparation:
[0039] Add glycerin, poloxamer, dextran, and calcium sodium edetate into 300ml of water for injection, dissolve, add sodium hydroxide to adjust the pH to 6.0-7.0, boil for 15 minutes, fill with nitrogen, cool and keep warm to 50°C.
[0040] Heat soybean oil for injection to 60°C, dissolve lecithin and clevidipine butyrate in soybean oil for injection under high shear at 7000rpm, keep warm at 50°C, and continuously fill with nitrogen during the preparation of the oil phase.
[0041] The water phase and the oil phase were sheared and mixed evenly, supplemented with water for injection to the formula quantity, and the high-pressure homogenization pressure was 700 bar, and homogenized until a fat emulsion with a particle size of less than 220nm was obtained.
[0042] Pass the above-mentioned fat emulsion through a 0.22 μm filter, filter and sterilize, fill in vials, place the filled and semi-stoppered semi-finished product ...
preparation Embodiment 2
[0044] prescription:
[0045]
[0046]
[0047] Preparation:
[0048] Add glycerin, sodium oleate, and sodium bisulfite to 300ml of water for injection, dissolve, add sodium hydroxide to adjust the pH to 8.0-9.0, boil for 15 minutes, fill with nitrogen, cool and keep warm to 55°C.
[0049] Heat soybean oil and medium-chain triglycerides for injection to 60°C, dissolve cholesterol and clevidipine butyrate in soybean oil and medium-chain triglycerides for injection under high shear at 8000rpm, and keep warm at 50°C , continuous nitrogen filling during oil phase preparation.
[0050] The water phase and the oil phase were sheared and mixed evenly, supplemented with water for injection to the formula amount, and the pressure of high-pressure homogenization was 600 bar, and homogenized until a fat emulsion with a particle size of less than 220nm was obtained, and lactose was added and stirred to dissolve.
[0051] Pass the above-mentioned fat emulsion through a 0.22 μm filt...
preparation Embodiment 3
[0053] prescription:
[0054]
[0055]
[0056] Preparation:
[0057]Add glycerin, poloxamer, mannitol, and vitamin C into 300ml of water for injection, dissolve, add sodium hydroxide to adjust the pH to 6.0-7.0, boil for 15 minutes, fill with nitrogen, cool and keep warm to 50°C.
[0058] Heat soybean oil for injection to 60°C, dissolve lecithin and clevidipine butyrate in soybean oil for injection under high shear at 7000rpm, keep warm at 50°C, and continuously fill with nitrogen during the preparation of the oil phase.
[0059] The water phase and the oil phase were sheared and mixed evenly, supplemented with water for injection to the formula quantity, and the high-pressure homogenization pressure was 700 bar, and homogenized until a fat emulsion with a particle size of less than 220nm was obtained.
[0060] Pass the above-mentioned fat emulsion through a 0.22 μm filter, filter and sterilize, fill in vials, place the filled and semi-stoppered semi-finished product o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com