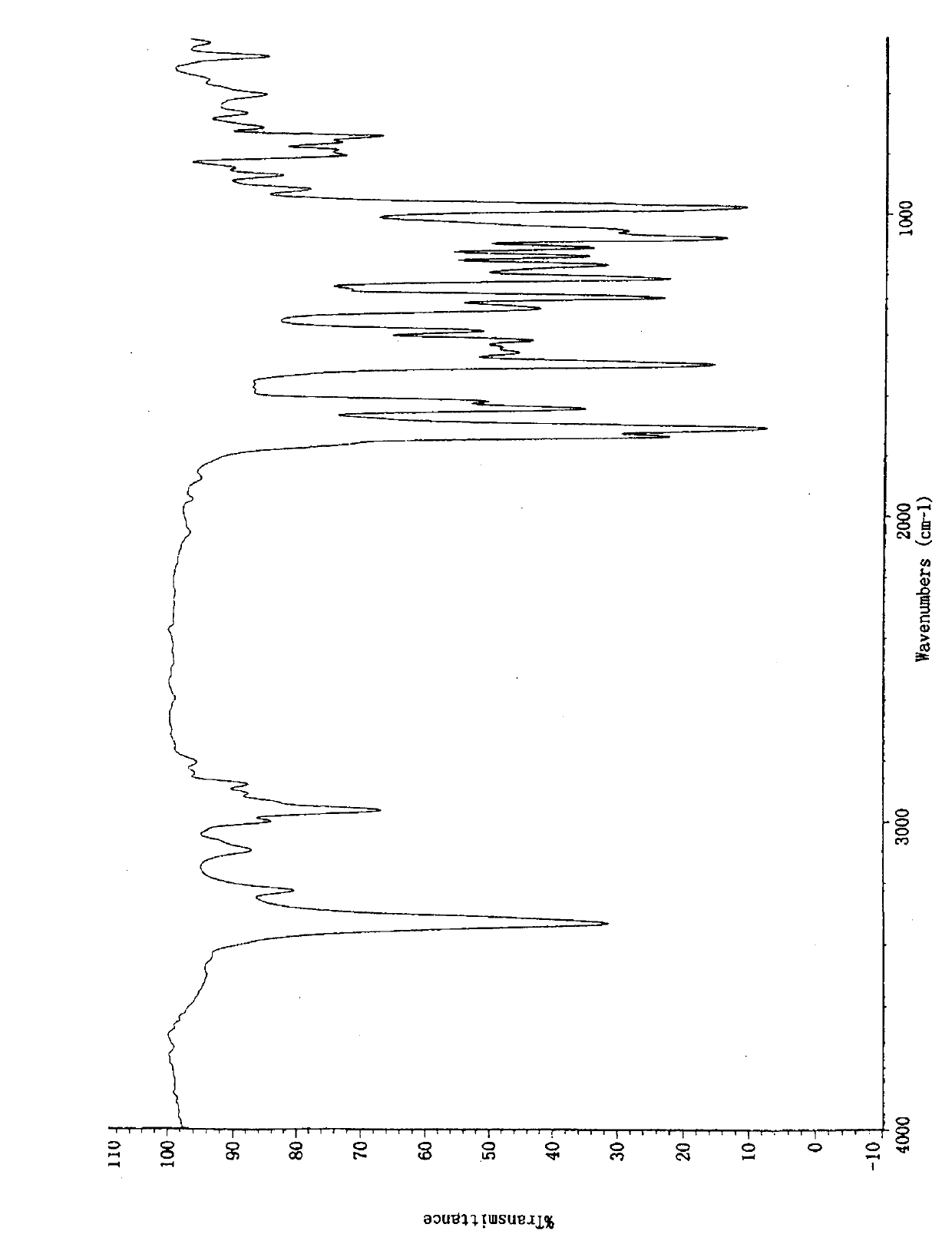
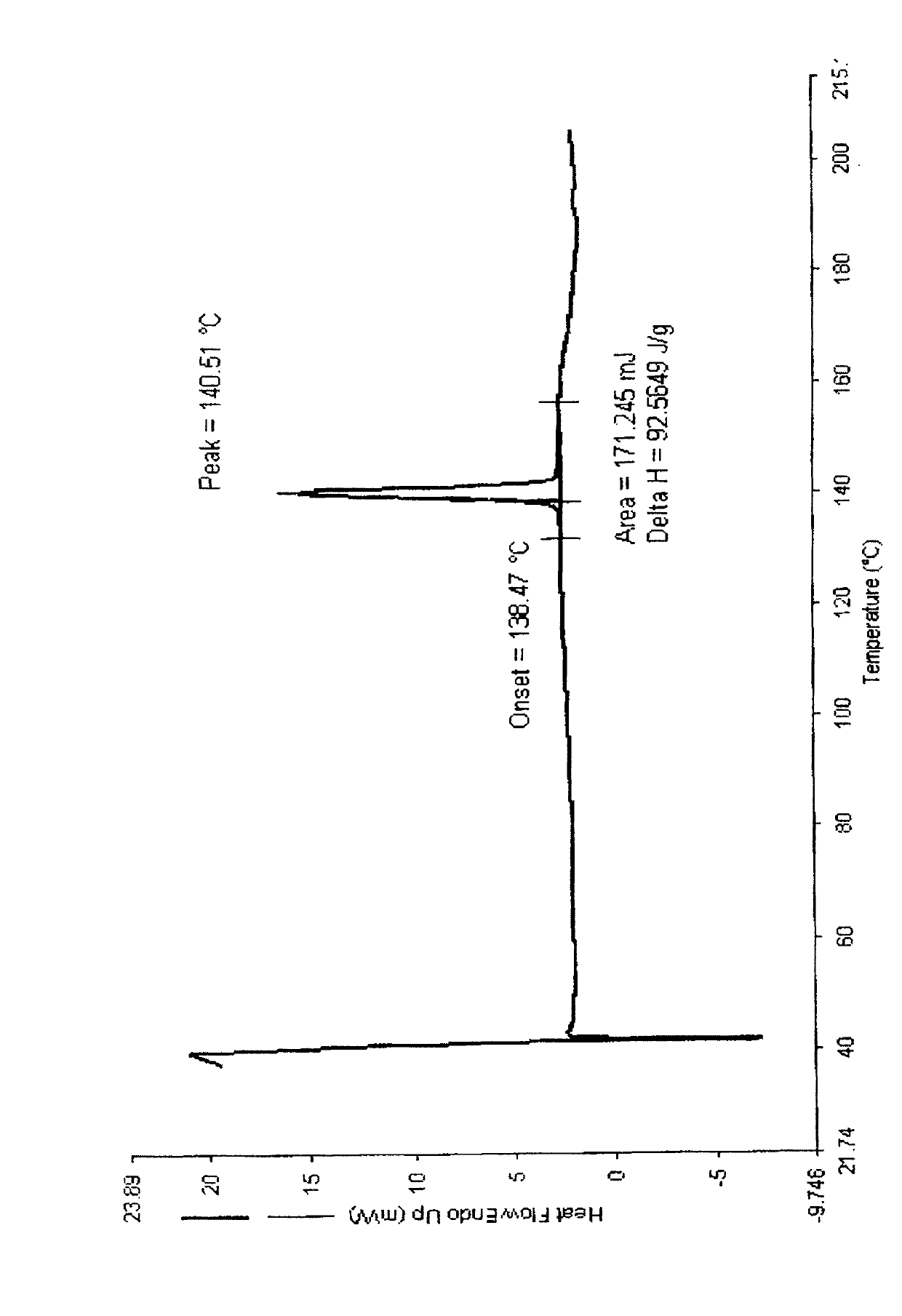
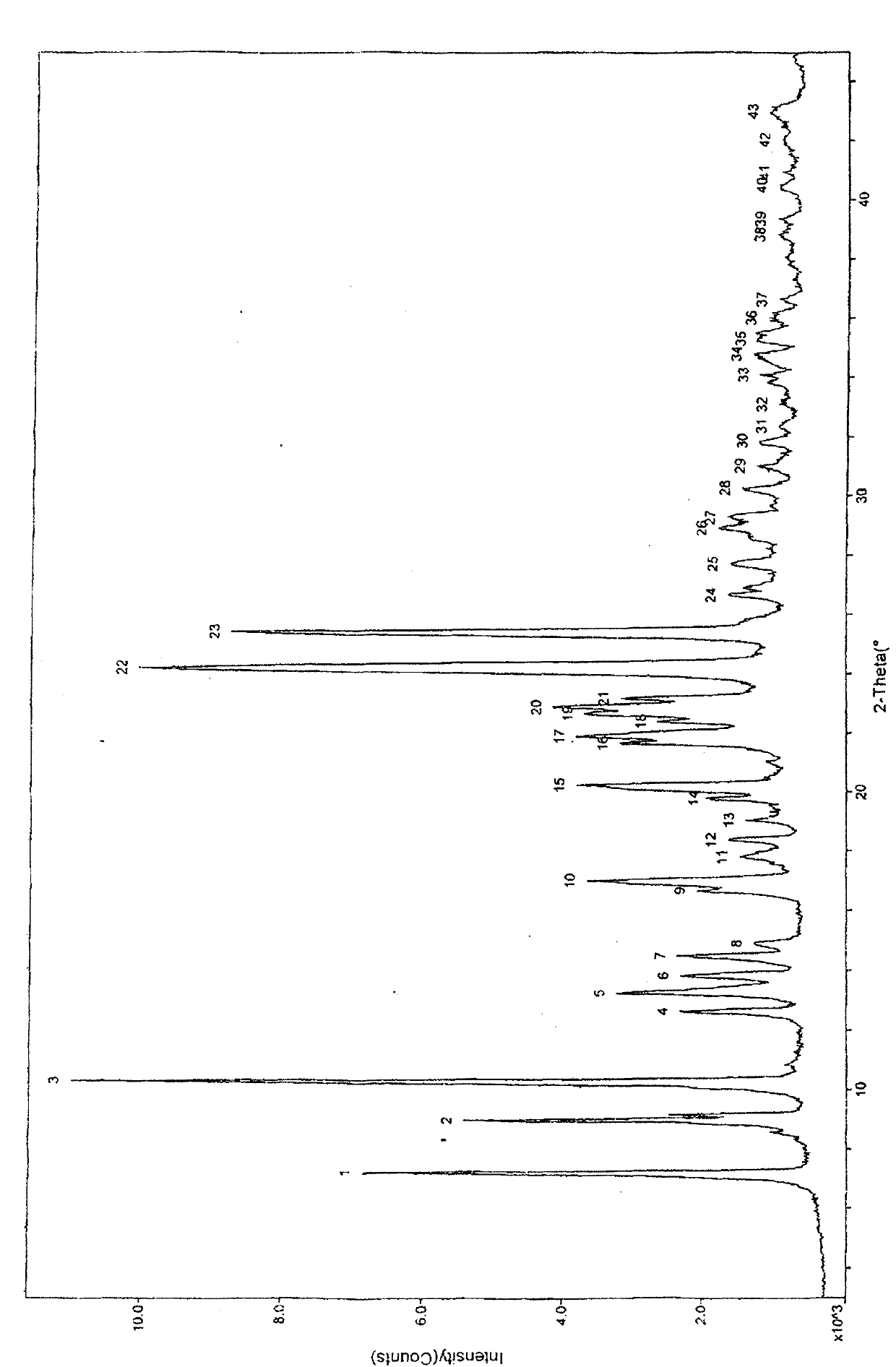
Crystal form of butyric acid clevidipine
A kind of technology of clevidipine butyrate and crystal form, applied in the field of medicine, can solve problems such as research report of clevidipine butyrate crystal form
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] The preparation of clevidipine butyrate I crystal form:
[0104] To 10 g of clevidipine butyrate solid, add 40 ml of isopropanol, heat to dissolve, add 0.5 g of activated carbon for decolorization, and filter while hot to obtain a filtrate. Add 60ml of water to the filtrate, keep the temperature at 20-25°C, stir for 6h, a white solid is precipitated, filtered, and dried to obtain crystal form I of clevidipine butyrate.
Embodiment 2
[0106] The preparation of clevidipine butyrate I crystal form:
[0107] To 10 g of clevidipine butyrate solid, add 45 ml of isopropanol, heat to dissolve, add 0.5 g of activated carbon for decolorization, and filter while hot to obtain a filtrate. Add 70ml of water to the filtrate, keep the temperature at 25-30°C, stir for 12h, a white solid is precipitated, filtered, and dried to obtain crystals of clevidipine butyrate in I crystal form.
Embodiment 3
[0109] The preparation of clevidipine butyrate I crystal form:
[0110] 10kg of clevidipine butyrate solid, add 32kg of isopropanol, heat to dissolve, add 500g of activated carbon for decolorization, filter while it is hot, add 60kg of water to the filtrate, add a small amount of clevidipine butyrate crystal seed of I crystal form, keep the temperature at 21- After stirring for 9 hours at 25°C, a white solid was precipitated, centrifuged, and dried to obtain Form I crystals of clevidipine butyrate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com