Purification method of clevidipine butyrate
A technology of clevidipine butyrate and a purification method, which is applied in the field of purification of clevidipine butyrate, can solve the problems that the inorganic impurities of clevidipine butyrate exceed the standard, etc., and achieve the effect of easy industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
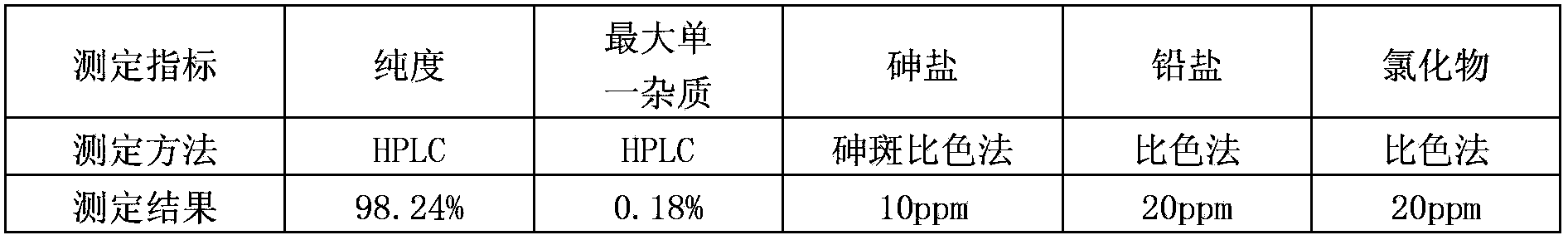
[0032] The crude product of clevidipine butyrate obtained by conventional method reaction is a filler with octadecylsilane bonded silica gel; acetonitrile-methanol-water (25:48:27) is mobile phase; the detection wavelength is 238nm, and the measured purity is 98.24%; the largest single impurity is 0.18%; the arsenic salt is measured by the arsenic spot colorimetric method, and the arsenic salt is 10ppm; the lead salt is measured by the heavy metal colorimetric method, and the lead salt is 20ppm; the standard solution colorimetric method is used to measure Chloride 20ppm. The experimental results are shown in Table 1:
[0033] Table 1
[0034]
[0035] Experimental results show: the clevidipine butyrate crude product used in the present invention has a purity of 98.24% after testing, and a total impurity of 1.76%, a purity lower than 99.5%; the largest single impurity is 0.18%, exceeding the required 0.1%; arsenic Salt is 10ppm, greater than 2ppm required for injection; le...
experiment example 2
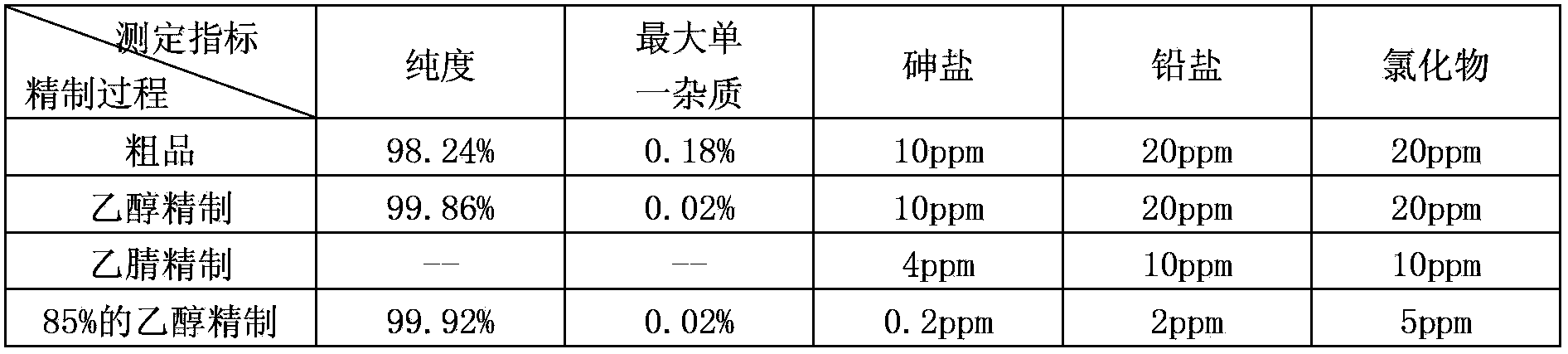
[0037] The clevidipine butyrate crude product and the product purity, the largest single impurity, arsenic salt, lead salt, and chloride determination results are shown in Table 2 after the method of embodiment 1 is refined:
[0038] Table 2
[0039]
[0040] The experimental results show that: through the above experiments, it can be seen that after refining with ethanol and acetonitrile, a product with a purity greater than 99.5% is obtained; after refining with 85% ethanol, arsenic salts, lead salts, chlorides, etc. are significantly reduced. The purity of the obtained clevidipine butyrate product is 99.92%, and the largest single impurity is 0.02%; the arsenic salt is 0.2ppm measured by atomic absorption, the lead salt is less than 2ppm, and the chloride is less than 5ppm.
experiment example 3
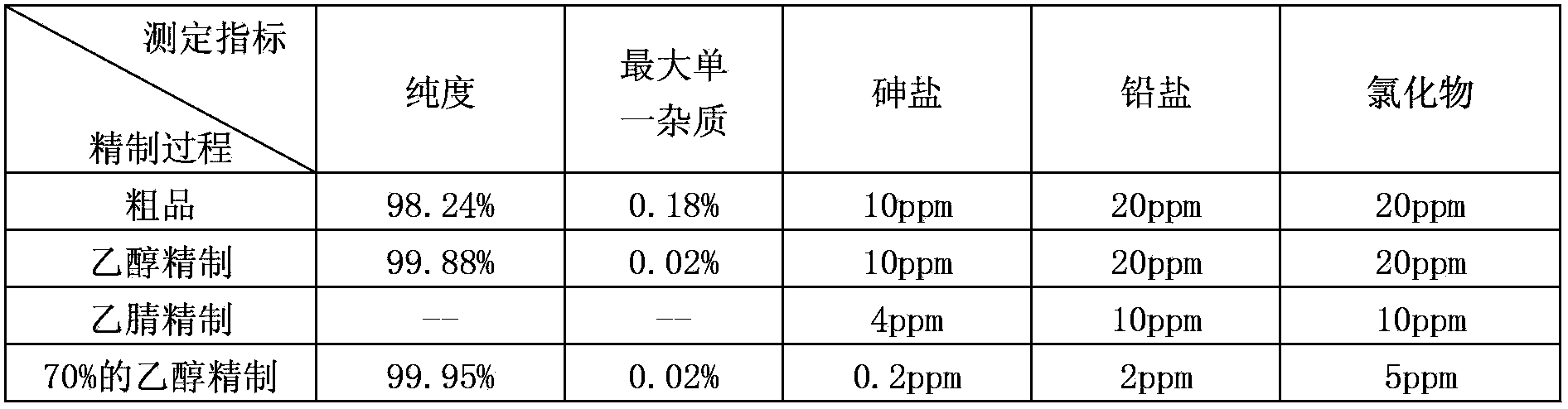
[0042] The clevidipine butyrate crude product and the product purity, maximum single impurity, arsenic salt, lead salt, and chloride determination results are shown in Table 3 through the method for embodiment 2 refining:
[0043] table 3
[0044]
[0045] The experimental results show that: through the above experiments, it can be seen that after refining with ethanol and acetonitrile, a product with a purity greater than 99.5% is obtained; after refining with 85% ethanol, arsenic salts, lead salts, chlorides, etc. are significantly reduced. The purity of the obtained clevidipine butyrate product is 99.95%, and the largest single impurity is 0.02%; the arsenic salt is 0.2ppm measured by atomic absorption, the lead salt is less than 2ppm, and the chloride is less than 5ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com