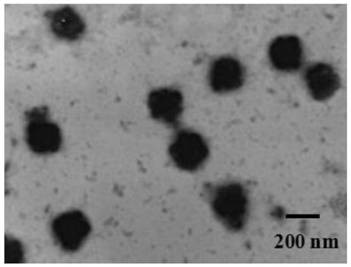
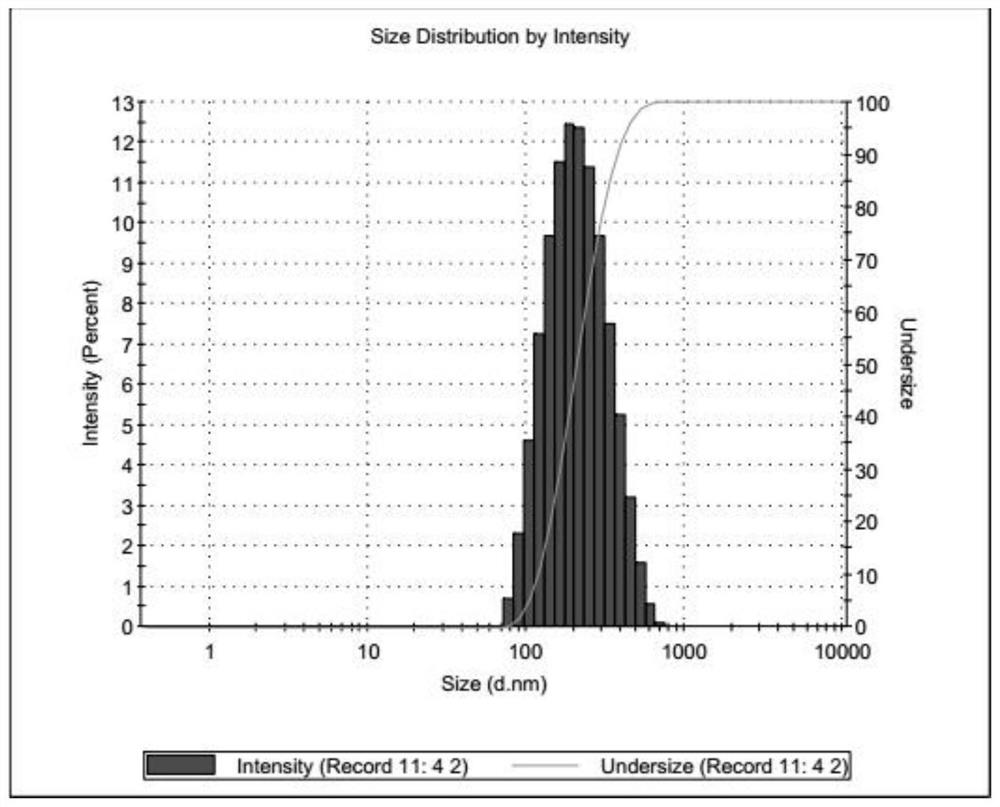
A kind of artemether liposome for injection and its preparation method and application
A technology of artemether and liposomes, which is applied in the field of artemether liposomes for injection and its preparation, and can solve the problems of leakage of contents, easy leakage of contents, short validity period, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] In yet another specific embodiment of the present invention, a method for preparing artemether liposomes is disclosed, which specifically includes the following steps:
[0050] Dissolve artemether, soybean lecithin, and cholesterol in absolute ethanol in proportion to ultrasonic dissolution to make a lipid solution, and then inject the lipid solution into phosphate buffered saline (PBS) at 40-45°C. After the injection is completed, Stir to remove ethanol, and then perform ultrasonic treatment to filter the membrane to obtain the artemether liposome liquid preparation.
[0051] In another specific embodiment of the present invention, the mass volume ratio of artemether, soybean lecithin, cholesterol, absolute ethanol and phosphate buffer is 1:6-8:1-2:0.3-0.4:0.8 -1.2(g:g:g:L:L);
[0052] In another specific embodiment of the present invention, the mass volume ratio of the artemether, soybean lecithin, cholesterol, absolute ethanol and phosphate buffer is 1:6:1.5:0.3:1.2...
Embodiment 1
[0059]
[0060] Preparation Process:
[0061] Dissolve the prescribed amount of artemether, soybean lecithin, and cholesterol with 3ml of absolute ethanol 40KHz ultrasound, mix well, slowly inject the mixed solution into 12ml of PBS heated to 45°C with a micro-injector, and use an electronic constant speed The stirrer was stirred at a speed of 800 rpm until the anhydrous ethanol was completely removed, and the resulting suspension was subjected to ultrasound (40KHz) and then passed through a 0.22 μm microporous membrane to obtain artemether liposomes.
Embodiment 2
[0063]
[0064] Preparation Process:
[0065] Dissolve the prescribed amount of artemether, soybean lecithin, and cholesterol with 4ml of absolute ethanol 30KHz ultrasound, mix well, slowly inject the mixed solution into 10ml of PBS heated to 45°C with a micro-injector, and use an electronic constant speed The stirrer was stirred at a speed of 700 rpm until the anhydrous ethanol was completely removed, and the resulting suspension was subjected to ultrasound (40KHz) and then passed through a 0.22 μm microporous membrane to obtain artemether liposomes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com