Antihypertensive drug fat emulsion injection and preparation method thereof
A technology of emulsion injection and fat, which is applied in the field of antihypertensive drug fat emulsion injection and its preparation, can solve the problems of needing strengthening and poor stability, and achieve the effect of stable quality and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
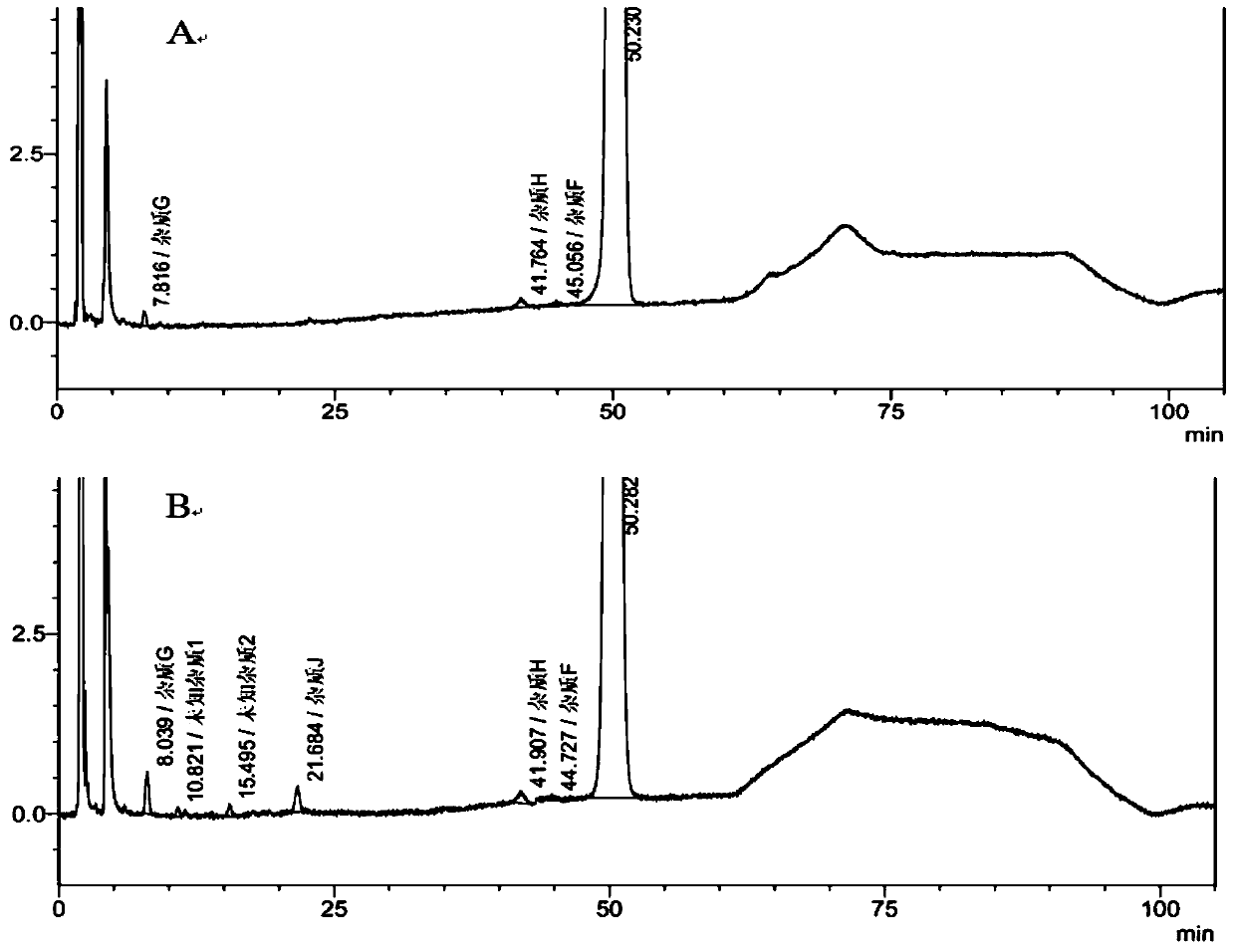
[0177] Example 1 Clevidipine Butyrate Fat Emulsion Injection Prescription Screening
[0178] The inventor sets the prescription composition of clevidipine butyrate fat emulsion injection of the present invention as follows: clevidipine butyrate, oil phase medium, emulsifier, osmotic pressure regulator, stabilizer, metal chelating agent, pH value adjustment medicaments, and water for injections. Furthermore, the specific prescription of clevidipine butyrate fat emulsion injection was screened, as follows:
[0179] 1. Prescription screening and basic process:
[0180] (1) Take the mixture of soybean oil for injection and medium-chain triglycerides (the weight ratio of the two is 1:1) and heat it to 70°C~75°C, add egg yolk lecithin, stabilizer, clevidipine butyrate, mix , shear until all are dissolved and dispersed evenly, so as to obtain the first mixture containing clevidipine butyrate oil phase.
[0181] (2) Heat water for injection to 70°C, add glycerin for injection and s...
Embodiment 2
[0206] The preparation of embodiment 2 clevidipine butyrate fat emulsion injection
[0207] prescription:
[0208]
[0209] Preparation method:
[0210] (1) Take the mixture of soybean oil for injection and medium-chain triglycerides (the weight ratio of the two is 1:1) and heat it to 70°C~75°C, add egg yolk lecithin E-80, phosphatidylglycerol, and butyric acid chloride Vidipine, mixed, sheared until completely dissolved and dispersed evenly, so as to obtain the first mixture containing clevidipine butyrate oil phase.
[0211] (2) Heat water for injection to 70°C, add glycerin for injection and stir evenly, add disodium edetate and stir until dissolved, so as to obtain the second mixture forming an aqueous phase.
[0212] (3) Mix the first mixture and the second mixture, and shear while mixing, at a rotation speed of 8000 rpm, for 10 minutes, so as to obtain a third mixture forming colostrum.
[0213] (4) Use 1 mol / L sodium hydroxide solution to adjust the pH of the thir...
Embodiment 3
[0228] The preparation of embodiment 3 clevidipine butyrate fat emulsion injection
[0229] prescription:
[0230]
[0231] Preparation method:
[0232] (1) Take the mixture of soybean oil for injection and medium-chain triglycerides (the weight ratio of the two is 1:1) and heat it to 70°C~75°C, add egg yolk lecithin E-80, phosphatidylglycerol, and butyric acid chloride Vidipine, mixed, sheared until completely dissolved and dispersed evenly, so as to obtain the first mixture containing clevidipine butyrate oil phase.
[0233] (2) Heat water for injection to 70°C, add glycerin for injection and stir evenly, add disodium edetate and stir until dissolved, so as to obtain the second mixture forming an aqueous phase.
[0234] (3) Mix the first mixture and the second mixture, and shear while mixing, at a rotation speed of 2000 rpm, for 30 minutes, so as to obtain a third mixture forming colostrum.
[0235] (4) Use 1 mol / L sodium hydroxide solution to adjust the pH of the thir...
PUM
| Property | Measurement | Unit |
|---|---|---|
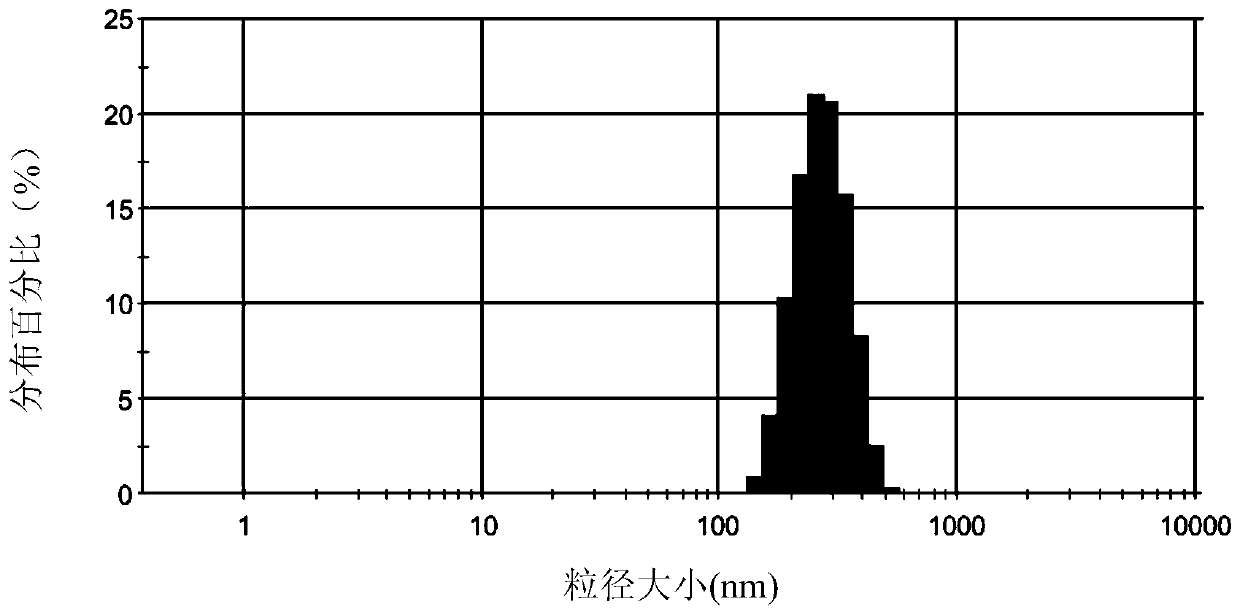
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com