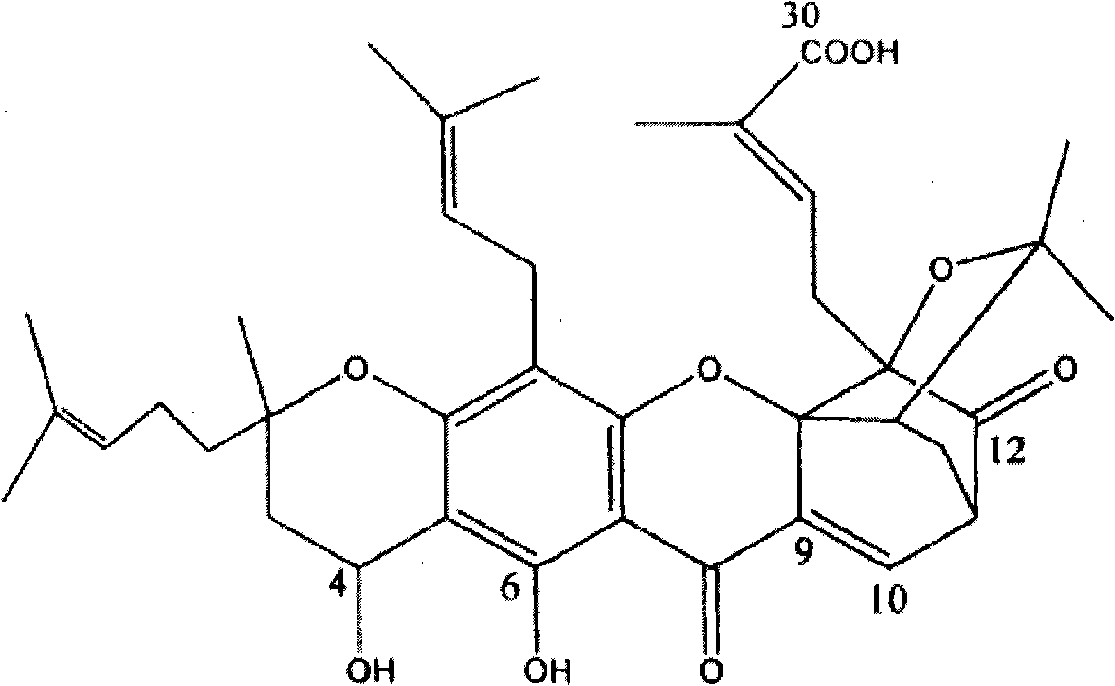
Neo-gambogic acid SLN (solid lipid nanoparticle) and preparation method thereof
A technology of solid lipid nanoparticles and lipid nanoparticles, which is applied in liquid transportation, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problems of large blood vessel irritation, low solubility, short half-life, etc., and achieve improved treatment The effect of index, mature preparation process and low solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Dissolve 10mg neogambogic acid and 100mg monostearic acid in chloroform, dissolve 200mg lecithin with a small amount of ethanol, and mix the two parts again to form the organic phase. Dissolve 300mg of poloxamer-188 and 2ml of glycerol in water for injection to form the aqueous phase. Slowly drop the organic phase at 70-80°C into the water phase at the same temperature (70-80°C), and stir at constant temperature until a transparent system is formed. The organic solvent was evaporated under reduced pressure with a rotary evaporator. The concentrated system is quickly mixed in the water phase at 0-2° C., stirred and cooled for 2 hours to obtain a solid lipid nanoparticle suspension. Filter through a microporous membrane to remove large particles, and store in a sealed container at 4°C.
[0059] Detection: the average particle diameter of neotectinic acid solid lipid nanoparticles is 221.8nm, and the encapsulation efficiency is 87.31%.
Embodiment 2
[0061] Dissolve 15mg neogambogic acid and 120mg monostearic acid in n-hexane, dissolve 300mg lecithin with a small amount of ethanol, and mix the two parts again to form the organic phase. Dissolve 400mg of poloxamer-188 and 2ml of glycerol in water for injection to form the aqueous phase. Slowly drop the organic phase at 70-80°C into the water phase at the same temperature (70-80°C), and stir at constant temperature until a transparent system is formed. The organic solvent was evaporated under reduced pressure with a rotary evaporator. The concentrated system is quickly mixed in the water phase at 0-2° C., stirred and cooled for 2 hours to obtain a solid lipid nanoparticle suspension. Filter through a microporous membrane to remove large particles, and store in a sealed container at 4°C.
[0062] Detection: the average particle size of neotectinic acid solid lipid nanoparticles is 184.5nm, and the encapsulation efficiency is 83.47%.
Embodiment 3
[0064] Dissolve 10mg neogambogic acid and 100mg monostearic acid in chloroform, dissolve 200mg lecithin with a small amount of ethanol, and mix the two parts again to form the organic phase. Dissolve 300mg Tween-80 and 2g glycerol in water for injection to form the aqueous phase. Slowly drop the organic phase at 70-80°C into the water phase at the same temperature (70-80°C), and stir at constant temperature until a transparent system is formed. The organic solvent was evaporated under reduced pressure with a rotary evaporator. The concentrated system is quickly mixed in the water phase at 0-2° C., stirred and cooled for 2 hours to obtain a solid lipid nanoparticle suspension. Filter through a microporous membrane to remove large particles, and store in a sealed container at 4°C.
[0065] Detection: the average particle diameter of neotectin solid lipid nanoparticles is 193.6nm, and the encapsulation efficiency is 85.34%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com