Etoposide lipidosome and preparation method thereof
A technology of etoposide and posideside, which is applied in liposome delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problems of non-compliance with drug safety and industrial production, and improve the chemical stability of drugs Sexuality, increasing curative effect, increasing water solubility and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
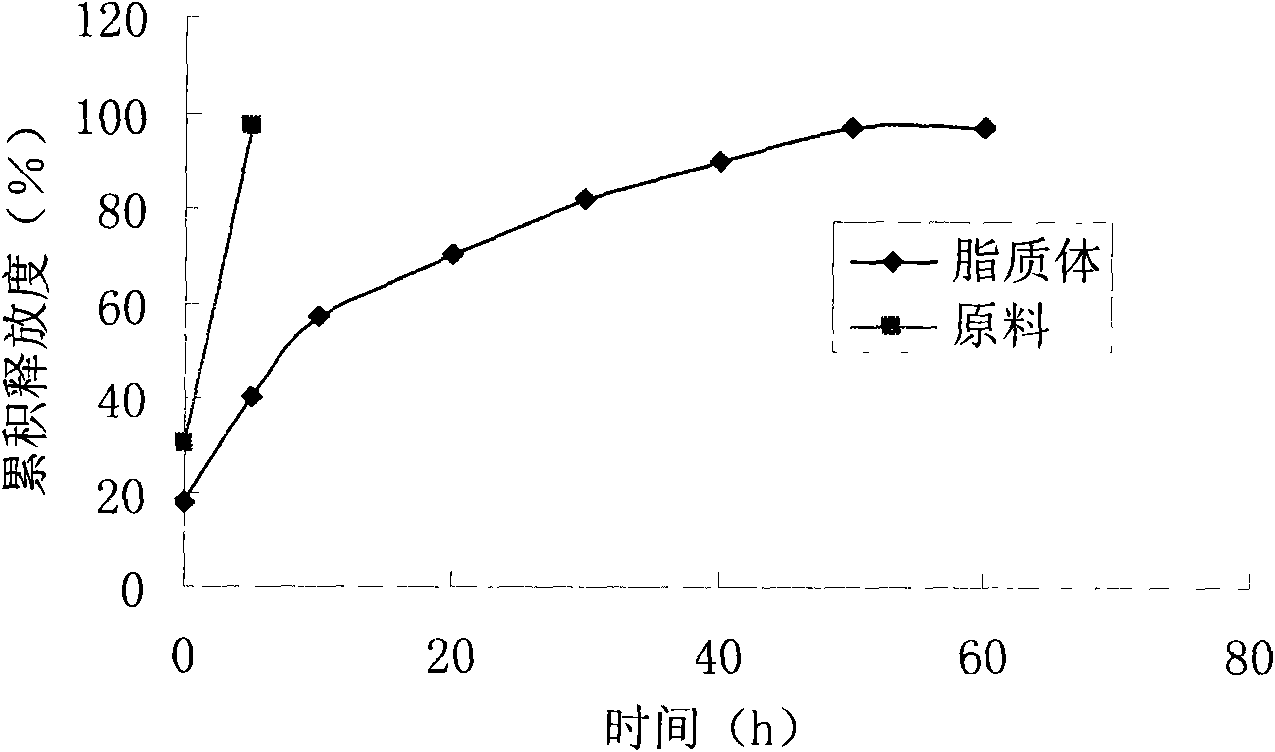
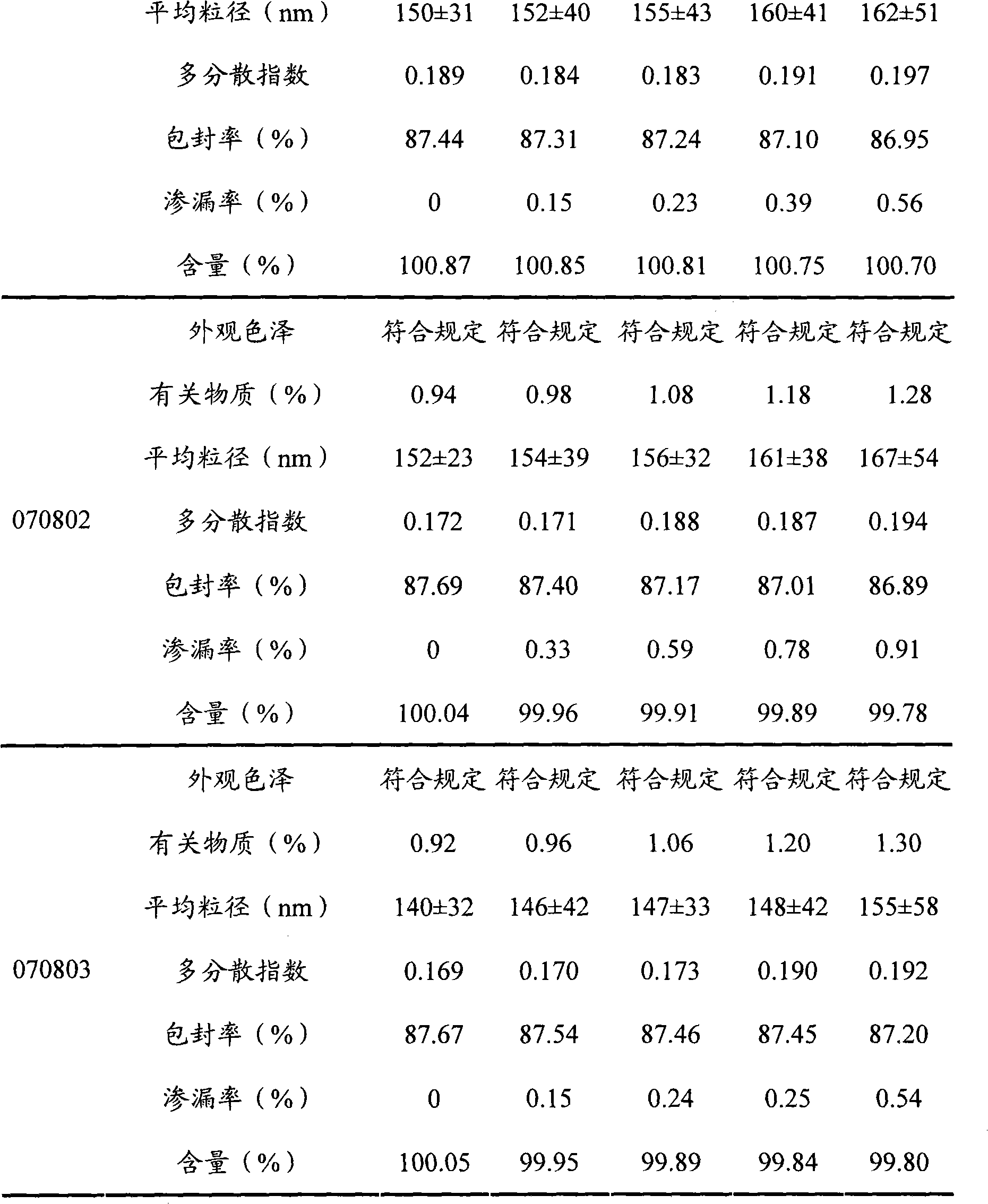
[0046] Take 100 mg of etoposide, 500 mg of lecithin, and 40 mg of cholesterol in a 50 ml eggplant-shaped bottle, add 20 ml of ethanol: acetone (2: 1) and ultrasonically dissolve it fully. Put the eggplant-shaped bottle in a 40°C constant temperature water bath to remove the organic solvent by rotary evaporation under reduced pressure, and form a layer of lipid film on the inner wall of the bottle, slowly add 10ml of phosphate buffer (pH7.0), hydrate, vortex, and make the eggplant The lipid film on the vial was completely dissolved. The mixture was ultrasonically dispersed at room temperature (ultrasonic power 600W) for 30 minutes to obtain etoposide liposomes. Its average particle size is 179nm, all particles are below 300nm, and the particle size distribution is narrow, indicating that the liposome size is relatively uniform; the liposome can be stable at room temperature for several days and at 4°C for at least 3 months, storage No precipitation or degradation of etoposide ...
Embodiment 2
[0048] Accurately weigh 100 mg of etoposide, 400 mg of soybean lecithin, 40 mg of cephalin, 30 mg of soybean steryl glucoside, and 20 mg of vitamin E, and dissolve them with 10 ml of ether. Then slowly inject this solution into 30ml of physiological saline (40°C) containing 0.5% sodium cholate with a syringe, stir with an electronic constant speed stirrer at a speed of 2000r / min until the ether is completely removed, and the mixed solution is homogenized under high pressure (homogeneous Pressure 10000psi) 5 times to obtain etoposide liposomes, which were stored in a refrigerator at 4°C for later use.
Embodiment 3
[0050] Accurately weigh 100mg of etoposide, 2000mg of hydrogenated lecithin, 800mg of soybean sterol, 50mg of cardiolipin, and 50mg of stearylamine in a Erlenmeyer flask, heat to melt, and place in a 75°C water bath for later use. Dissolve 100mg of poloxamer 188, 400mg of glycerin, and 100mg of vitamin C in 20ml of 5% glucose solution, heat to 75°C, add to the lipid melt, mix at high speed, and homogenize under high pressure (homogenization pressure 15000psi) 3 times to obtain etoposide liposomes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com