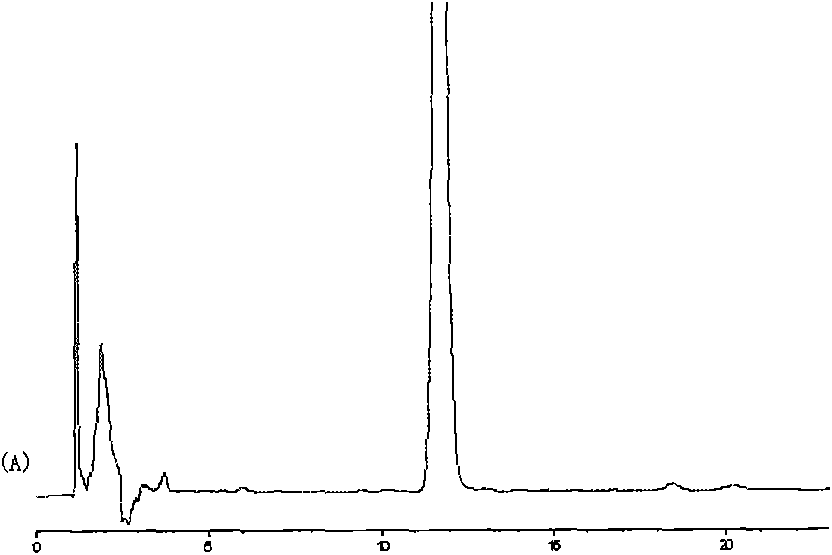
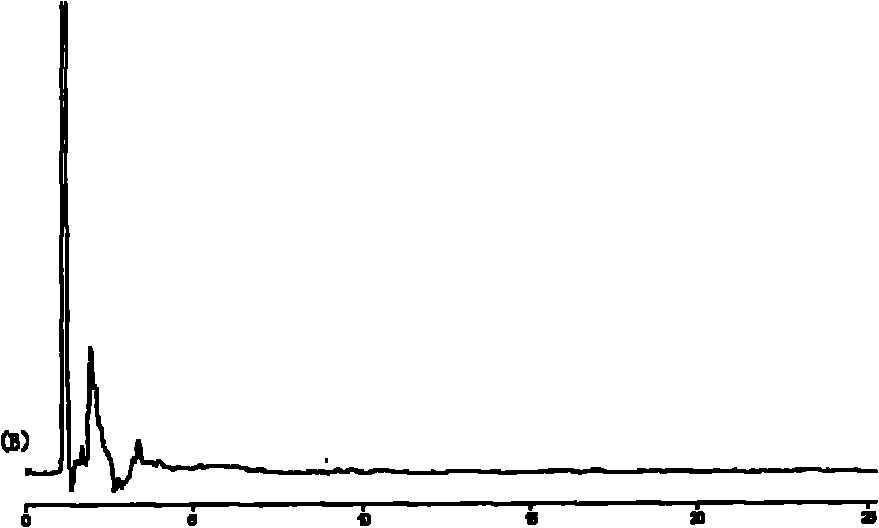
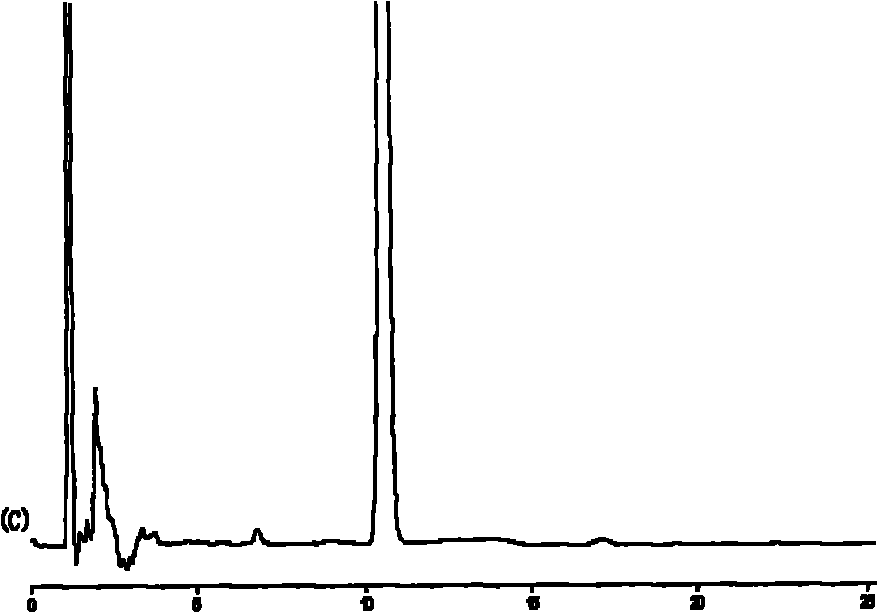
Taxol submicroemulsion taking cholesterol complex as intermediate carrier
A paclitaxel and cholesterol technology, which is applied in the directions of drug combination, drug delivery, emulsion delivery, etc., can solve the problems of not conforming to the principles of pharmacoeconomics, the cost of preparing submicron emulsions, and the cost of preparing submicron emulsions. Safety, reduced preparation cost, and low bile solids intake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1 paclitaxel cholesterol complex
[0051] A total of 6 groups of complexes were prepared, wherein the amount of cholesterol in complexes 1 and 2 was within the range required by the present invention, that is, the paclitaxel / cholesterol weight ratio was between 1:0.09 and 1:0.90; complexes 3 to 6 The amount of cholesterol is higher, beyond the range required by the present invention, specifically 1: 1.37 ~ 1: 9.06. Compounds 3 to 6 were used for subsequent emulsion encapsulation preparation and comparative study of encapsulation. The composition of the above 6 groups of paclitaxel / cholesterol complexes is shown in the table below.
[0052] Table 1: Composition of paclitaxel / cholesterol complex
[0053] Compound number
cholesterol
Paclitaxel / cholesterol (weight ratio)
Complex 1
8.0g
3.6g
1∶0.45
Complex 2
8.0g
7.2g
1∶0.90
Complex 3
3.0g
4.11g
1∶1.37
Comple...
Embodiment 2
[0056] Embodiment 2: Paclitaxel submicroemulsion with paclitaxel cholesterol complex as intermediate carrier
[0057] [Prescription Composition]
[0058]
[0059]
[0060] *Complex 1 is paclitaxel / cholesterol complex 1 prepared in Example 1.
[0061] [Preparation]
[0062] Take about 130-140ml of water for injection, add egg yolk lecithin, poloxamer (188) and glycerin according to the prescribed amount, disperse in a tissue grinder to make a uniform water phase, heat to 40°C, and keep warm;
[0063] Measure soybean oil according to the prescription, preheat it to 40°C, weigh the paclitaxel-cholesterol complex 1 prepared in Example 1, dissolve it in the preheated soybean oil, disperse it in a tissue grinder to form a uniform oil phase;
[0064] Under stirring conditions, slowly add the water phase to the oil phase, cut at 10,000 rpm for 5 min to form uniform colostrum, quickly transfer to a high-pressure homogenizer, homogenize 6 times, collect all the emulsion, and...
Embodiment 3
[0066] Embodiment 3: Paclitaxel submicroemulsion with paclitaxel cholesterol complex as intermediate carrier
[0067] [Prescription Composition]
[0068]
[0069] *Complex 2 is paclitaxel / cholesterol complex 2 prepared in Example 1.
[0070] [Preparation]
[0071] Take about 130-140ml of water for injection, add poloxamer (188) and glycerin according to the prescription amount, disperse in a tissue grinder to make a uniform water phase, heat to 80°C, and keep warm;
[0072] Measure soybean oil according to the prescription, preheat to 80°C, weigh compound 2 and egg yolk lecithin according to the prescription, add it to the preheated soybean oil, disperse and dissolve in a tissue masher to make a uniform and transparent oil Mutually;
[0073] Under stirring conditions, slowly add the water phase to the oil phase, cut at 20,000 rpm for 10 min to form uniform colostrum, quickly transfer to a high-pressure homogenizer, homogenize 6 times, collect all the emulsion, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com