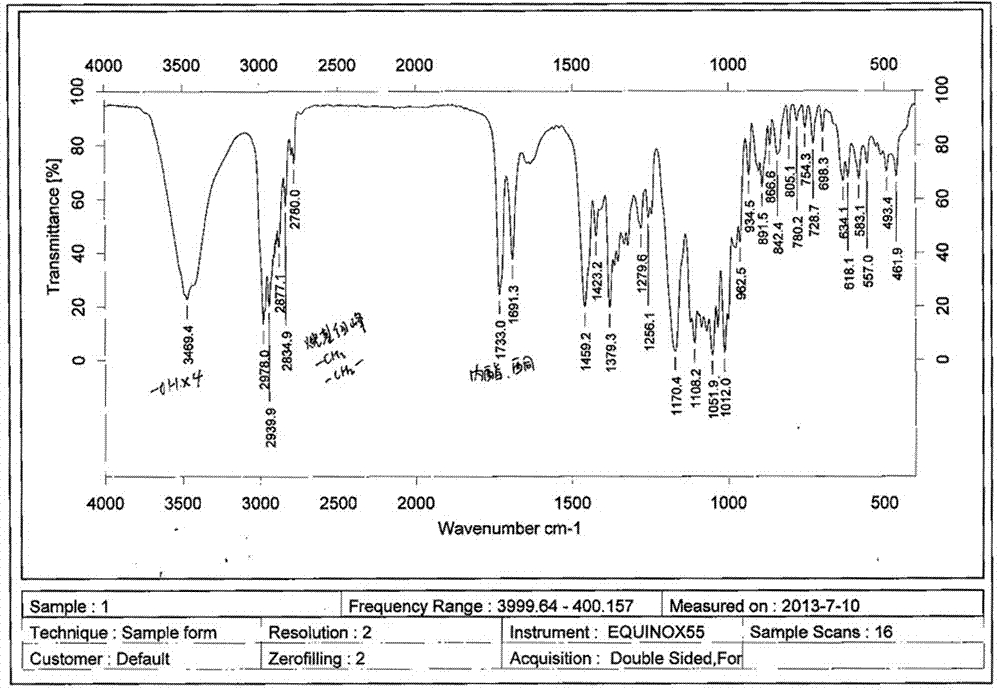
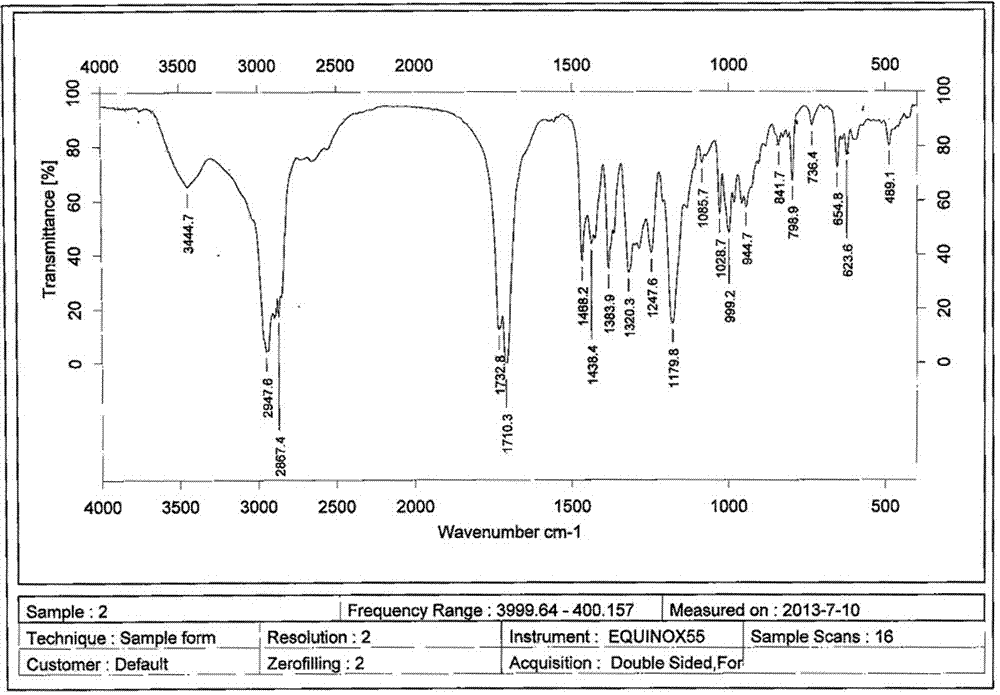
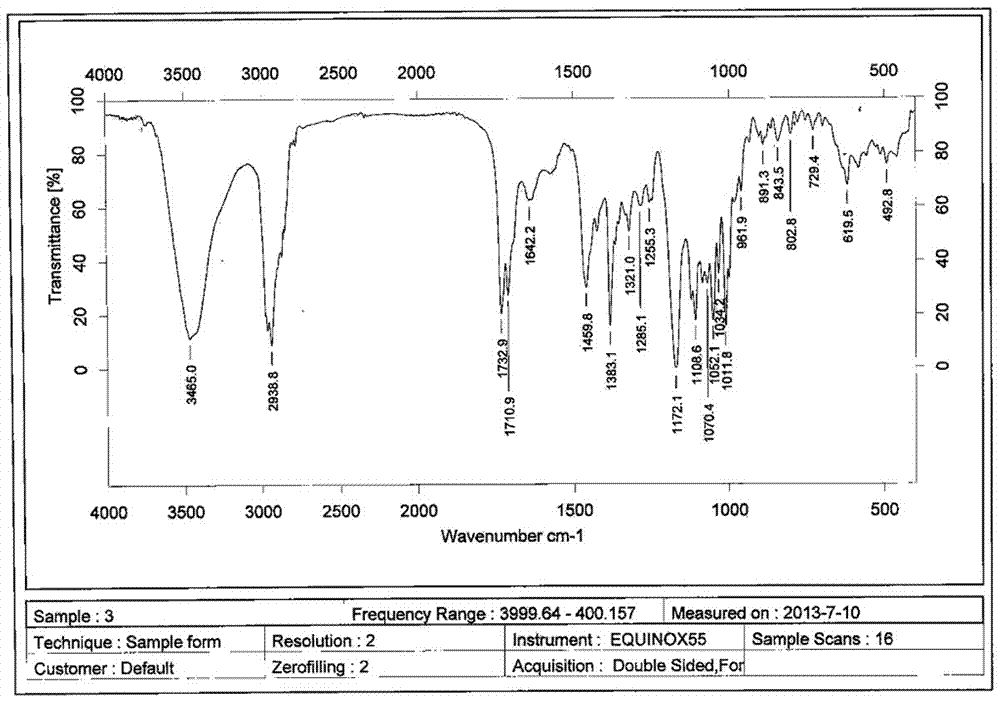
A clarithromycin ion-pair lipid microsphere injection and preparation method thereof
A technology of clarithromycin and lipid microspheres, applied in liposome delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problems of clarithromycin irritation, low solubility, and difficulty in development, etc. Achieve the effects of reducing vascular irritation, improving antibacterial activity, reducing pain and vascular irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Embodiment 1 formula 1 clarithromycin content 250mg: 100ml
[0094] Based on 100ml injection
[0095]
[0096] Preparation of clarithromycin ion-pair lipid microsphere injection:
[0097] Step 1: Disperse 2.25g of glycerin for injection and 0.2g of pluronic F-680 in an appropriate amount of water for injection, heat it to 60°C in a magnetic stirrer, and dissolve them completely to obtain an aqueous phase, and keep it warm at 60°C for later use;
[0098] Step 2: Dissolve 4 g of egg yolk lecithin, 0.25 g of clarithromycin, and 0.325 g of cholesterol succinate monoester with an appropriate amount of absolute ethanol, remove the ethanol by rotary evaporation, and blow dry with nitrogen. Hydrate the obtained phospholipid dry film with the aqueous phase prepared in step 1 at 60° C. for 30 min to obtain a clarithromycin liposome coarse dispersion system for subsequent use;
[0099] Step 3: Preheat 10gMCT at 60°C and use it as the oil phase for later use;
[0100] Step 4:...
Embodiment 2
[0103] Embodiment 2 formula 2 clarithromycin content 100mg: 100ml;
[0104] Based on 100ml injection
[0105]
[0106] Preparation of clarithromycin ion-pair lipid microsphere injection:
[0107] Step 1: Disperse 2.25g of glycerin for injection and 0.2g of pluronic F-680 in an appropriate amount of water for injection, heat it to 60°C in a magnetic stirrer, and dissolve them completely to obtain an aqueous phase, and keep it warm at 60°C for later use;
[0108] Step 2: Dissolve 4 g of egg yolk lecithin, 0.1 g of clarithromycin, and 0.13 g of cholesterol succinate monoester with an appropriate amount of absolute ethanol, remove the ethanol by rotary evaporation, and blow dry with nitrogen. Hydrate the obtained phospholipid dry film with the aqueous phase prepared in step 1 at 60° C. for 30 min to obtain a clarithromycin liposome coarse dispersion system for subsequent use;
[0109] Step 3: Preheat 10gMCT at 60°C and use it as the oil phase for later use;
[0110] Step 4: ...
Embodiment 3
[0113] Embodiment 3 formula 3 clarithromycin content 500mg: 100ml
[0114] Based on 100ml injection
[0115]
[0116] Preparation of clarithromycin ion-pair lipid microsphere injection:
[0117] Step 1: Disperse 2.25g of glycerin for injection and 0.2g of pluronic F-680 in an appropriate amount of water for injection, heat it to 60°C in a magnetic stirrer, and dissolve them completely to obtain an aqueous phase, and keep it warm at 60°C for later use;
[0118] Step 2: Dissolve 4 g of egg yolk lecithin, 0.5 g of clarithromycin, and 0.65 g of cholesterol succinate monoester with an appropriate amount of absolute ethanol, remove the ethanol by rotary evaporation, and blow dry with nitrogen. Hydrate the obtained phospholipid dry film with the aqueous phase prepared in step 1 at 60° C. for 30 min to obtain a clarithromycin liposome coarse dispersion system for subsequent use;
[0119] Step 3: Preheat 10gMCT at 60°C and use it as the oil phase for later use;
[0120] Step 4: u...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com