A kind of alprostadil freeze-dried microemulsion composition and preparation method thereof
A technology of alprostadil and freeze-dried microemulsion, which is applied in the field of alprostadil freeze-dried microemulsion composition and its preparation, can solve the problems of high content of degraded impurities, vascular stimulation, etc., achieve encapsulation efficiency and stability improvement, Avoid drug degradation and reduce the effect of vascular irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
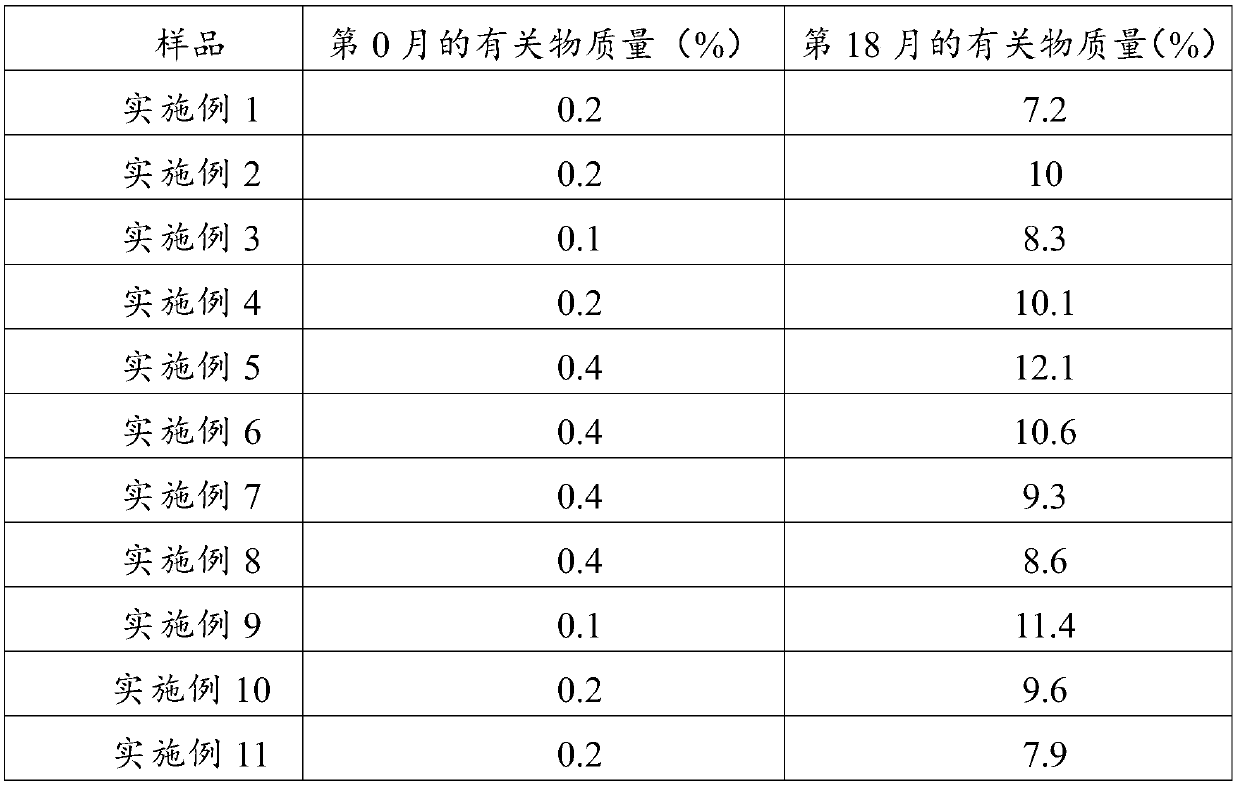
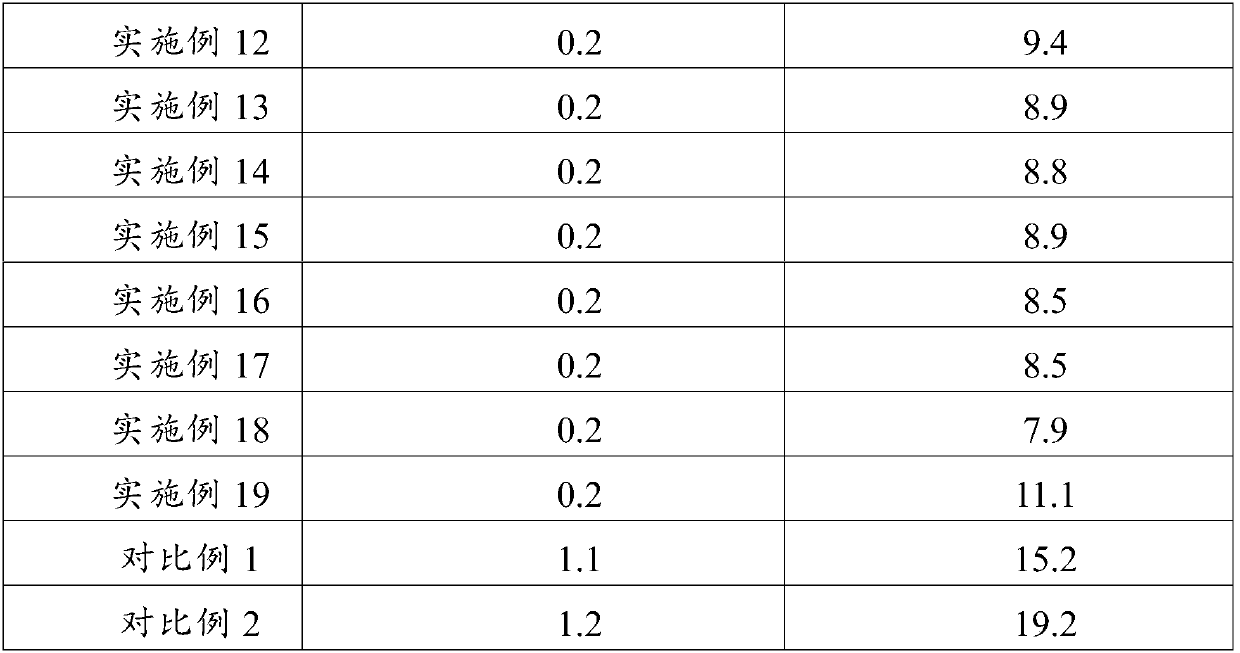
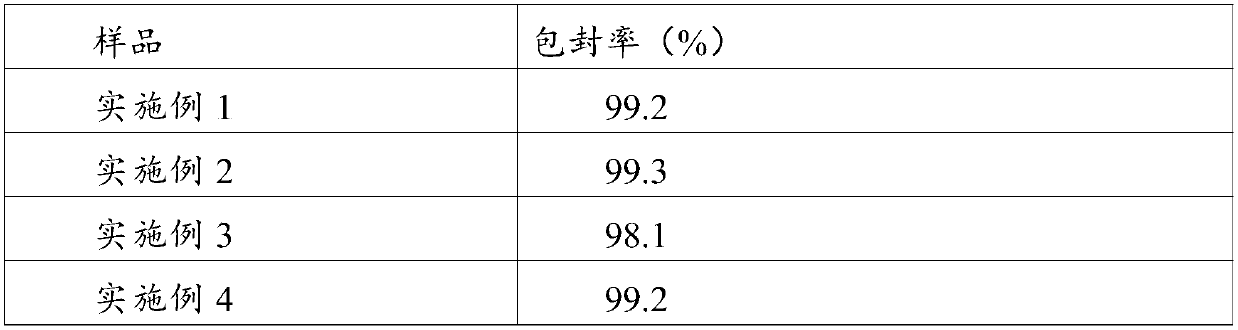
Examples
Embodiment 1
[0042] Embodiment 1: the preparation of composition of the present invention
[0043] Weigh 26g of polyethylene glycol 15-hydroxystearate, 2g of phosphatidylglycerol, 25g of medium-chain triglycerides, and 4g of phosphatidylcholine, stir at about 60°C to dissolve and mix evenly, then lower the temperature to 25°C Add 5 mg of alprostadil, stir to dissolve and mix evenly to obtain a mixed oil phase; weigh 75 g of glucose, add it to 600 ml of water, stir to dissolve, and control the temperature at about 25°C to obtain a water phase; Mix with mixed oil, control the temperature at about 25°C; adjust the pH to 5.2 with 0.5M hydrochloric acid and 0.5M sodium hydroxide, add water to the full amount (1L); homogenize 3 times under the pressure of 400bar through a high-pressure homogenizer, Control the temperature at about 8°C; filter aseptically, fill in vials, freeze-dry, cap, and package.
Embodiment 2
[0044] Embodiment 2: the preparation of composition of the present invention
[0045] Weigh 20g polysorbate 80, 3g phosphatidylserine, 20g medium chain triglycerides, 5g phosphatidylcholine, stir at about 50°C to dissolve and mix evenly, lower the temperature to about 29°C, add 5mg of alprostadil, Stir to dissolve and mix evenly to obtain a mixed oil phase; weigh 75g of sucrose, add it to 800ml of water, stir to dissolve, and control the temperature at about 29°C to obtain a water phase; under stirring, mix the water phase with the mixed oil phase, and control the temperature 29°C or so; use 0.5M hydrochloric acid and 0.5M sodium hydroxide to adjust the pH to 4.0, add water to the full amount (1L); use a high-pressure homogenizer to homogenize twice under a pressure of 400bar, and control the temperature at about 10°C; Bacterial filtration, filled in vials, freeze-dried, capped, packaged, ready to be obtained.
Embodiment 3
[0046] Embodiment 3: the preparation of composition of the present invention
[0047] Weigh 15g of polyethylene glycol 15-hydroxystearate, 1g of phosphatidylinositol, 10g of medium-chain triglycerides, and 5g of phosphatidylcholine, stir at about 65°C to dissolve and mix evenly, and lower the temperature to 22°C Add 5 mg of alprostadil, stir to dissolve and mix evenly to obtain a mixed oil phase; weigh 50 g of mannitol, add it to 500 ml of water, stir to dissolve, and control the temperature at about 22°C to obtain a water phase; Mix the phase with the mixed oil phase, control the temperature at about 20°C; adjust the pH to 5.0 with 0.5M hydrochloric acid and 0.5M sodium hydroxide, add water to the full amount (1L); homogenize 6 times under the pressure of 100bar through a high-pressure homogenizer , temperature controlled at about 8°C; after aseptic filtration, filled in vials, freeze-dried, capped, and packaged, ready to be obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com