A kind of aerosol for treating chronic obstructive pulmonary disease
A chronic obstructive, aerosol technology, applied in the field of medicine, can solve the problems of little effect of disease progression, limited therapeutic effect, side effects, etc., to avoid clinical risks, improve symptomatic treatment efficacy, and improve the effect of lesions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
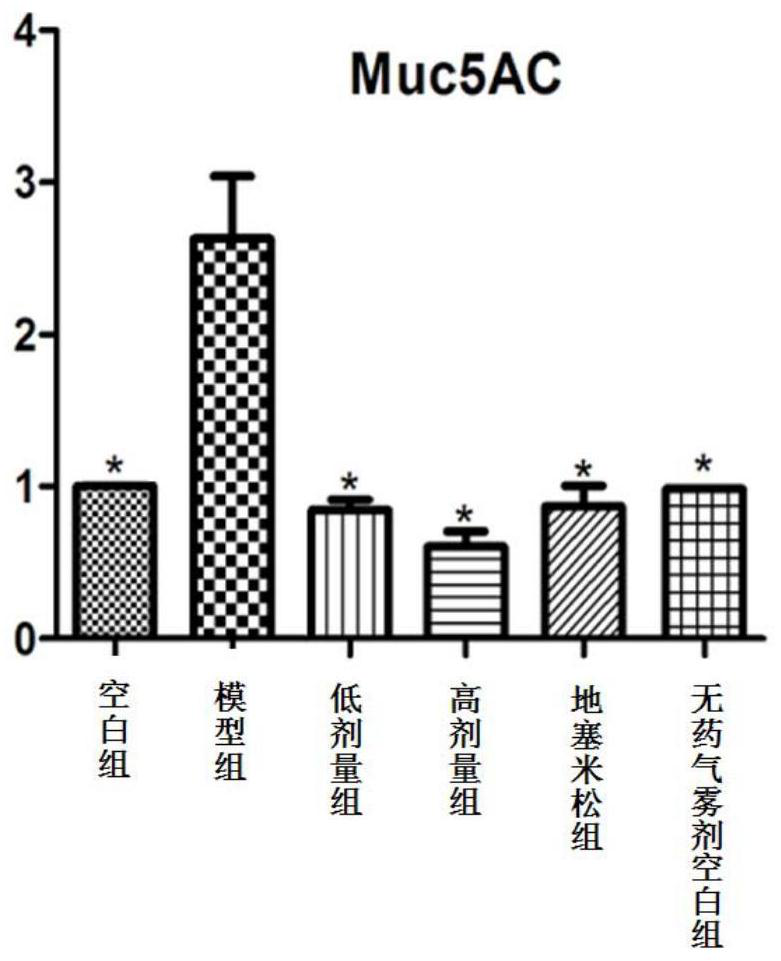
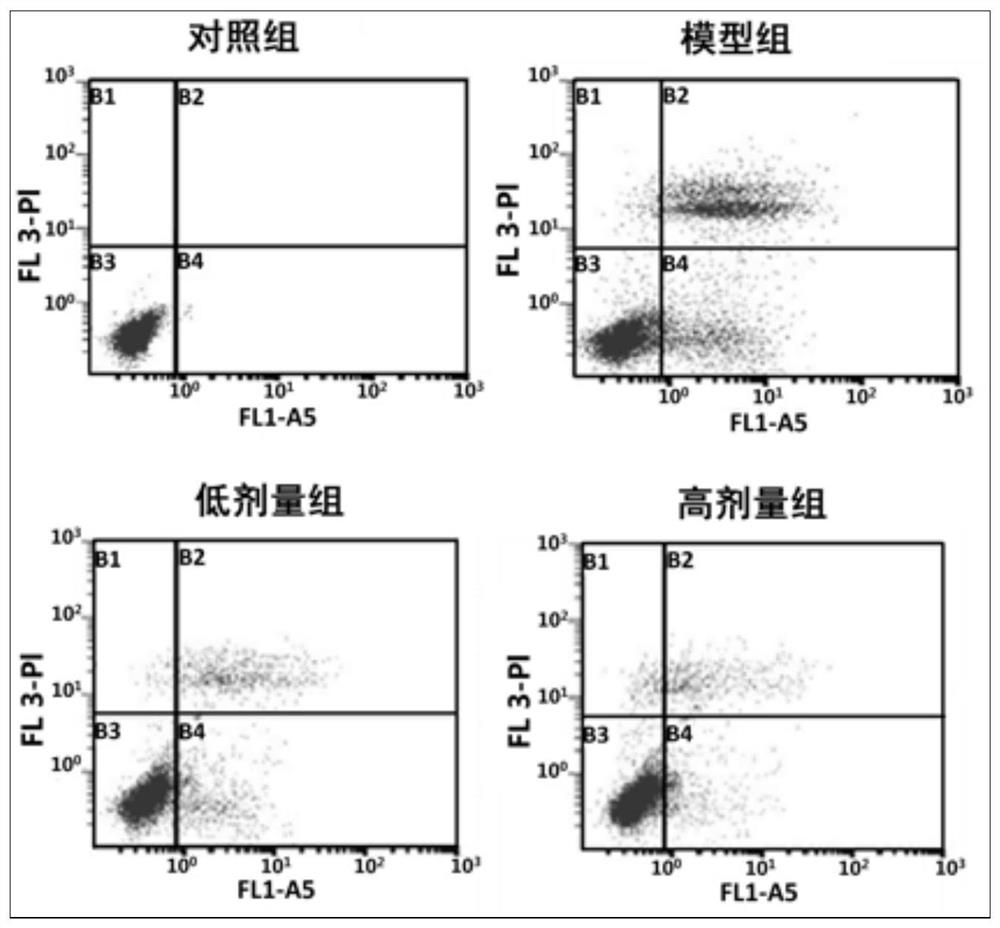
Image
Examples
Embodiment 1
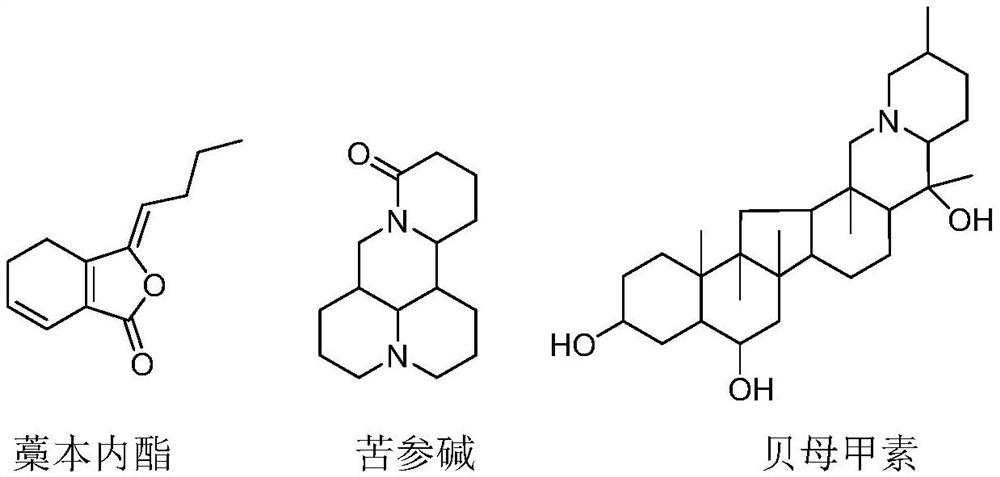
[0022] Mix ligustilide: matrine: peiminin according to the mass ratio of 1%:5%:94%, a total of 8000mg, add additives absolute ethanol, propylene glycol, Tween-80 each 80mL, 20mL and 1g, Form 100mL solution, pour it into 100 bottles of 25mL aerosol pressure bottles on average, each bottle is 1mL, use an aerosol filling machine to press 12g of propellant R134a into each bottle, 100 bottles in total.
Embodiment 2
[0024] Mix ligustilide: matrine: peiminin according to the mass ratio of 55%: 20%: 25%, a total of 4000mg, add additives absolute ethanol, propylene glycol, polyethylene glycol, each 70mL, 30mL and 0.5g , to form a 100mL solution, pour it into 100 bottles of 20mL aerosol pressure bottles on average, each bottle is 1mL, and use an aerosol filling machine to press 10g of propellant dimethyl ether into each bottle, a total of 100 bottles.
Embodiment 3
[0026] Ligustilide: Matrine: Peiminin are mixed according to mass ratio 63%: 18%: 19%, 10g in total, add 1.2g of talcum powder, 5g of oleic acid, 10g of anhydrous calcium chloride, 20g of lactose, The above-mentioned solids are mixed evenly, passed through a 400-500 mesh sieve, and packed into 100 bottles, and 11g of propellant heptafluoropropane is pressed into each bottle with an aerosol filling machine, a total of 100 bottles.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com