Establishment method for HPLC (high performance liquid chromatography) fingerprint of cold treatment medicament
A technology for establishing methods and fingerprints, applied in measuring devices, instruments, scientific instruments, etc., can solve the problem that the content of effective active ingredients cannot represent the curative effect, and achieve the effects of easy realization of chromatographic conditions, good precision, and simple production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
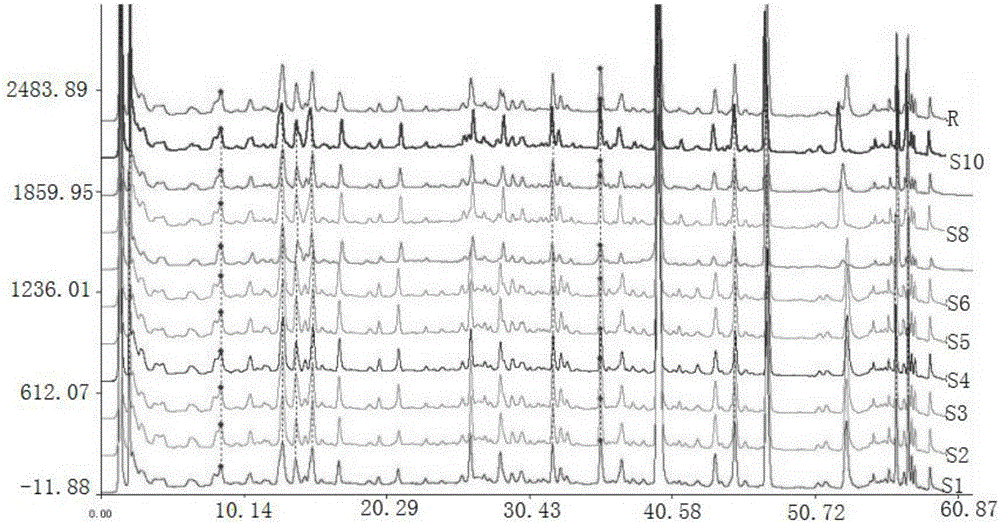
Image
Examples
Embodiment 1
[0043] 1 Instruments and reagents
[0044] 1.1 Instrument
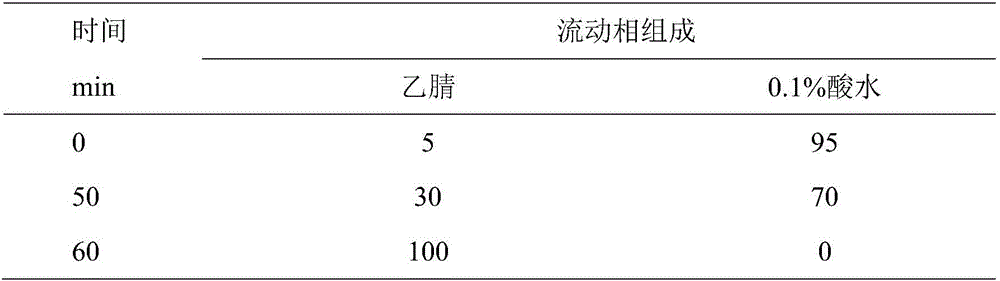
[0045] Waters 2695 high performance liquid chromatography, Waters 2998 detector, Waters Empower chemical workstation; Traditional Chinese medicine chromatographic fingerprint similarity evaluation system (version 2004A); BUG25-12 ultrasonic cleaning machine (220W, 50KHZ, Shanghai Branson Ultrasonic Co., Ltd.); AEG-45SM electronic balance (1 / 100,000, Mettler, Switzerland), BP121S (1 / 10,000, Beijing Sartorius Scientific Instrument Co., Ltd.).
[0046] 1.2 Reagent
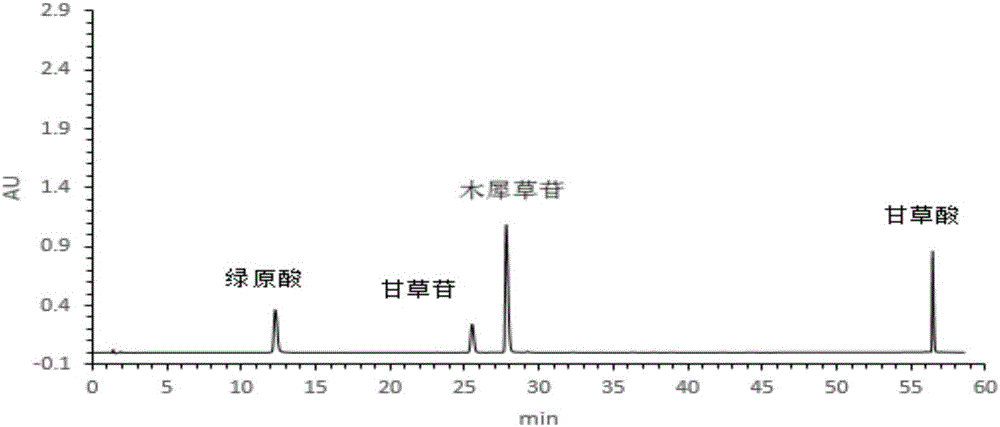
[0047] All reagents are analytically pure except that the mobile phase is chromatographically pure; glycyrrhizic acid reference substance (Beijing Hengyuan Qitian Chemical Technology Research Institute, batch number MUST-13083101); liquiritin reference substance (Beijing Hengyuan Qitian Chemical Technology Research Institute) Institute, batch number MUST-130020901); chlorogenic acid reference substance (Beijing Hengyuan Qitian Chemical Technology Research I...
Embodiment 2
[0090] A method for establishing the HPLC fingerprint of a cold medicine, the steps of which are the same as in Example 1.
[0091]The raw materials of cold medicine are: ephedra 6g, mint 5g, cicada slough 5g, honeysuckle 15g, scutellaria baicalensis 12g, bitter almond 9g, Fritillaria 6g, bellflower 6g, licorice 5g; preparation method: mint steam distillation extracts volatile oil, distilled The aqueous solution is stored in another container; the dregs and the remaining ephedra and other eight flavors are decocted with water for 1-3 times, each time for 0.5-1.5 hours, adding 6-10 times the weight of water for the first time, adding 5-8 times the weight of water for the second or third time, Combine the decoction, filter, combine the filtrate with the above aqueous solution, and concentrate to a clear paste with a relative density of 1.18 to 1.20 at a relative density of 90 to 95°C; take the clear paste, add 1-3 parts by weight of sucrose, and 1-3 parts by weight of dextrin, T...
Embodiment 3
[0093] A method for establishing the HPLC fingerprint of a cold medicine, the steps of which are the same as in Example 1.
[0094] The raw materials of cold medicine are: ephedra 3g, mint 9g, cicada slough 3g, honeysuckle 17g, scutellaria baicalensis 9g, bitter almond 16g, Fritillaria 3g, bellflower 8g, licorice 4g; The aqueous solution is stored in another container; the dregs and the remaining ephedra and other eight flavors are decocted with water for 1-3 times, each time for 0.5-1.5 hours, adding 6-10 times the weight of water for the first time, adding 5-8 times the weight of water for the second or third time, Combine the decoction, filter, combine the filtrate with the above aqueous solution, concentrate to a clear paste with a relative density of 1.18-1.20 at a relative density of 90-95°C; the obtained volatile oil is encapsulated with an appropriate amount of β-cyclodextrin, and an appropriate amount of clear paste is added for coloring, and sprayed Drying; take the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com