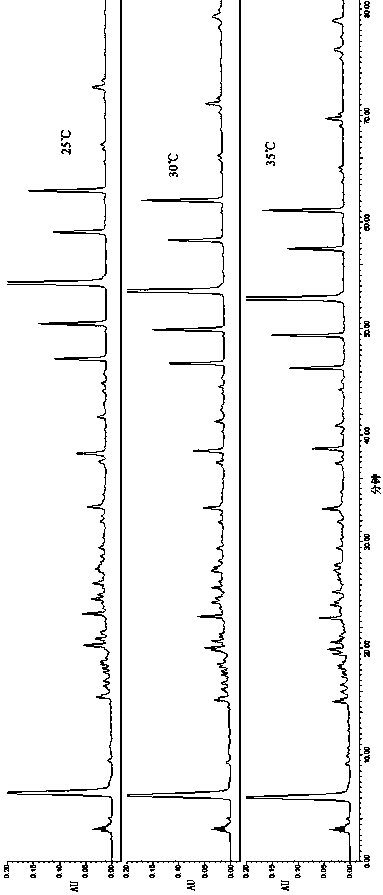
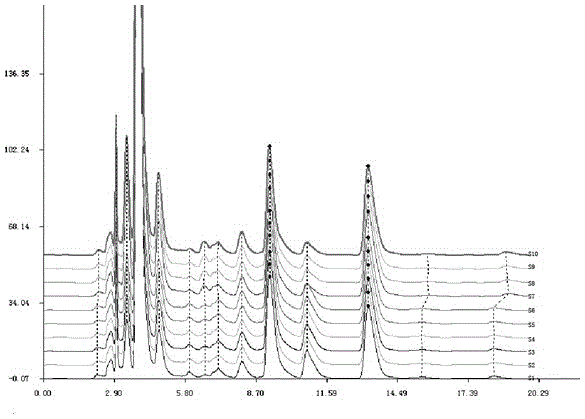
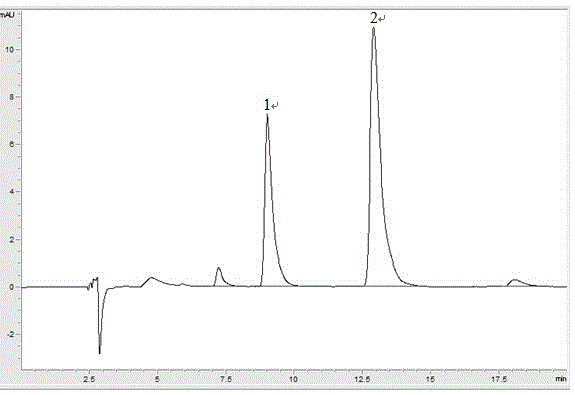
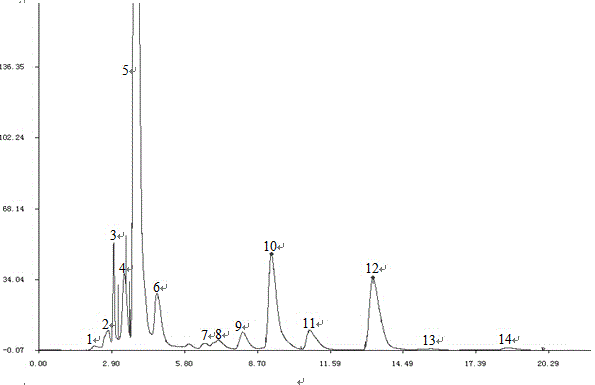
Patents
Literature
67results about How to "Chromatographic conditions are easy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Licorice medicinal materials fingerprint establishment method and its standard fingerprint
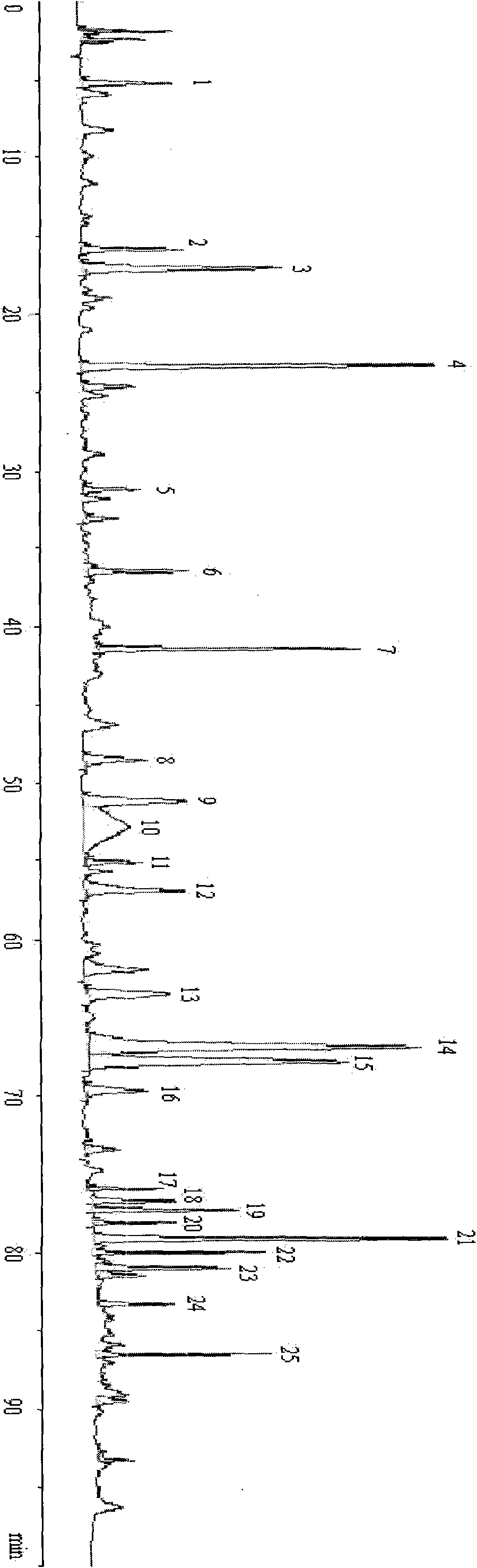
InactiveCN101216465AEasy to makeChromatographic conditions are easy to achieveComponent separationTesting medicinal preparationsChemistryChromatography column
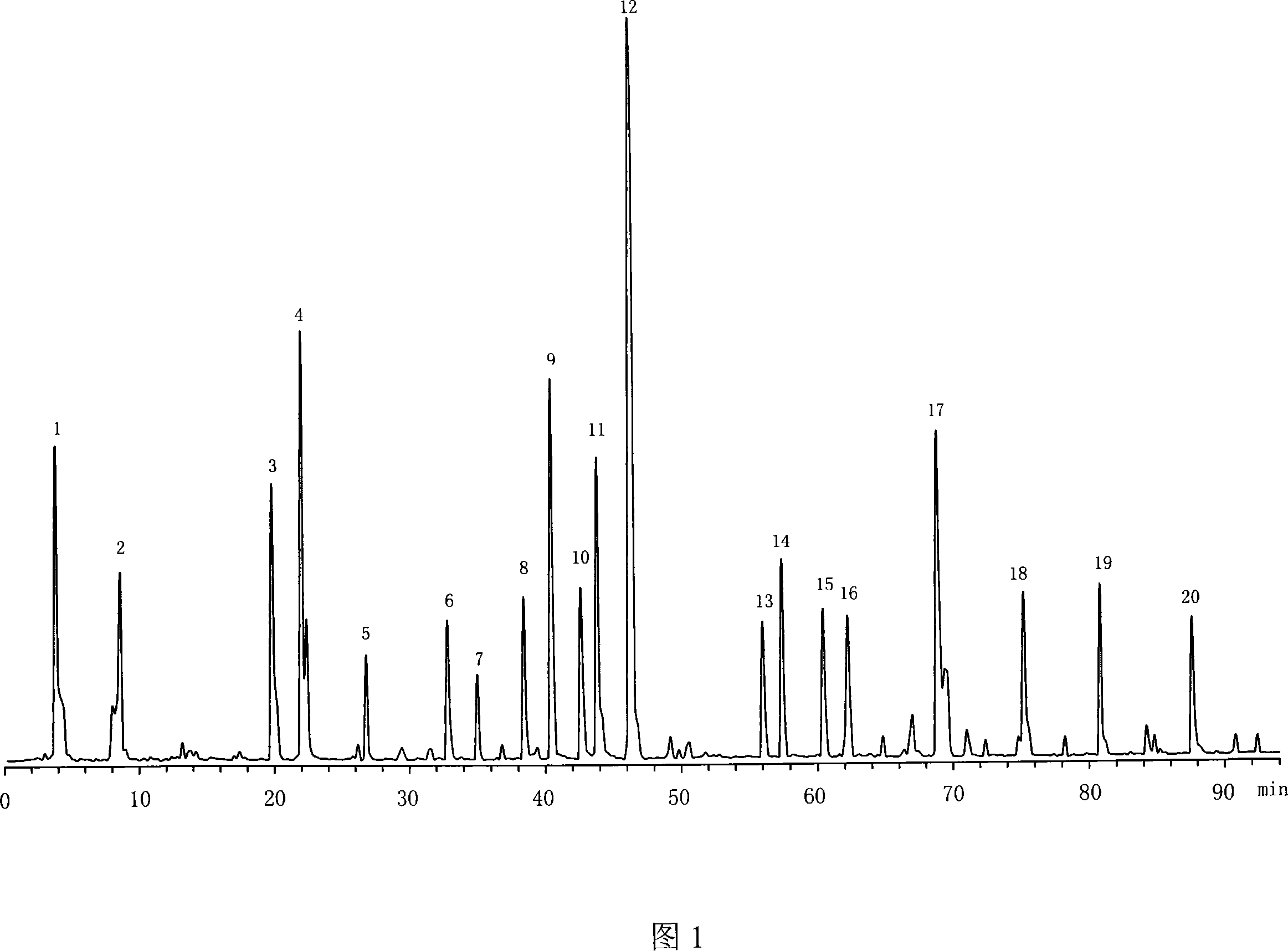
The invention provides a method for establishing an HPLC fingerprint spectrum of Radix Glycyrrhizae and a standard fingerprint spectrum thereof. The method comprises the following steps of: firstly adding Radix Glycyrrhizae powder into a 70% ethanol aqueous solution, ultrasound-extracting, filtering the supernate with a microporous membrane to obtain a filtrate as the test solution; then carrying out HPLC analysis, wherein the chromatographic column is Kromasil-C18 (5 mum, 250*4.6 mm), the mobile phase is 0.8% acetic acid solution-acetonitrile, the gradual eluting mode is adopted, the detection wavelength is 254 nm and the column temperature is 30 DEG C; and extracting the test solution, injecting into the HPLC to obtain the HPLC fingerprint spectrum of Radix Glycyrrhizae. The standard fingerprint spectrum of Radix Glycyrrhizae can be obtained by comparing HPLC fingerprint spectra of 16 batches of Radix Glycyrrhizae materials and determining the common fingerprint characteristics. The standard fingerprint spectrum can be used for effectively monitoring the quality of Radix Glycyrrhizae.
Owner:BIOCHEM ENG COLLEGE OF BEIJING UNION UNIV
Medlar HPLC fingerprint establishment method and its standard fingerprint
InactiveCN101216466ASignificant advantagesSignificant useComponent separationTesting medicinal preparationsHplc fingerprintComputer science
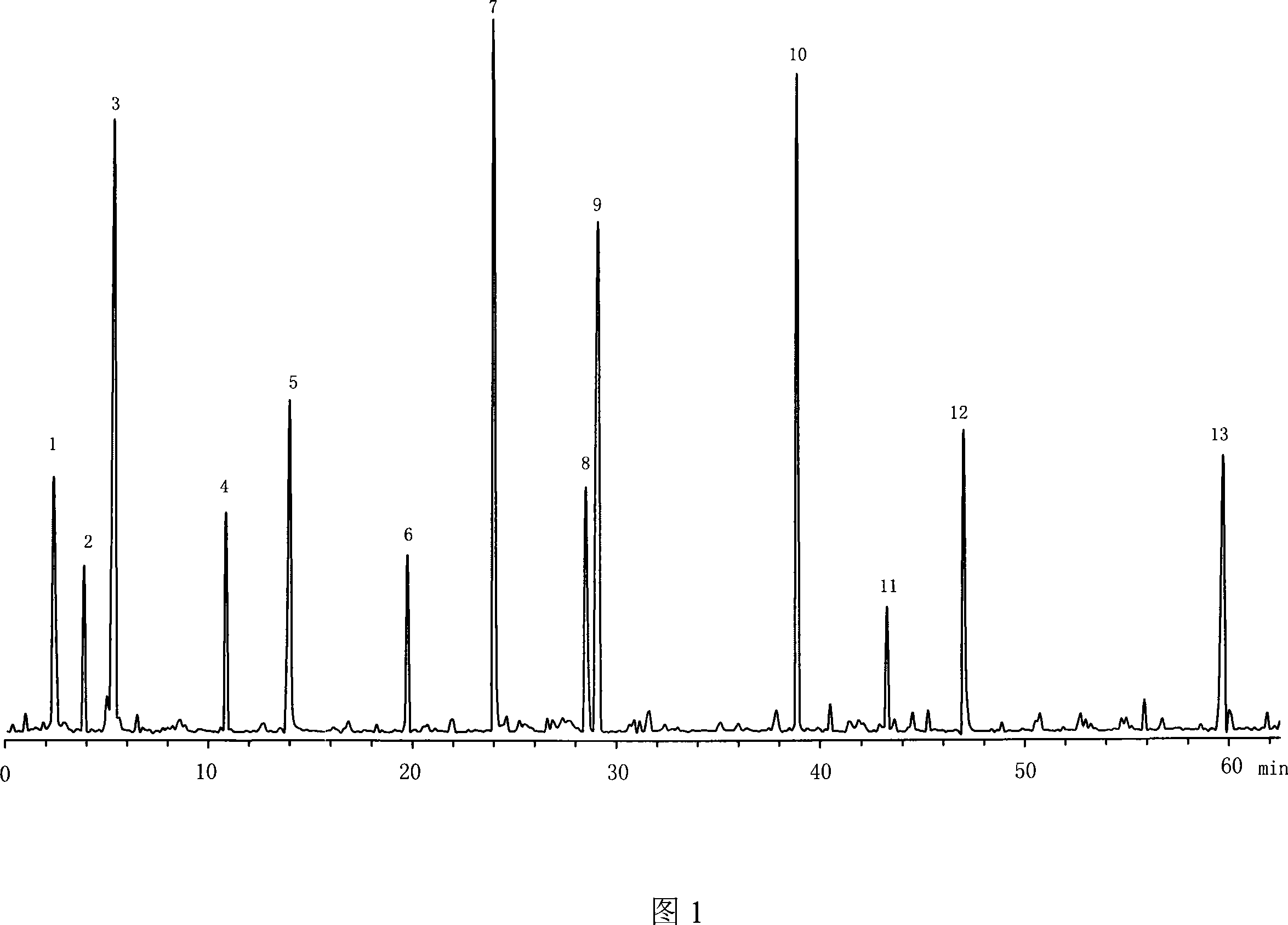
The invention provides a method for establishing an HPLC fingerprint spectrum of Fructus Lycii and a standard fingerprint spectrum thereof. The method comprises the following steps of: preparing a test solution, preparing a reference solution, selecting chromatographic conditions, and carrying out HPLC determination to obtain the fingerprint spectrum of Fructus Lycii. The standard fingerprint spectrum of Fructus Lycii can be obtained by comparing HPLC fingerprint spectra of 21 batches of Fructus Lycii materials and determining the common fingerprint characteristics. The method has the advantages of simple operation, good reproducibility, a plurality of characteristic peaks, accurate and reliable result, etc. The standard fingerprint spectrum can be used for effectively monitoring the quality of Fructus Lycii.
Owner:BIOCHEM ENG COLLEGE OF BEIJING UNION UNIV
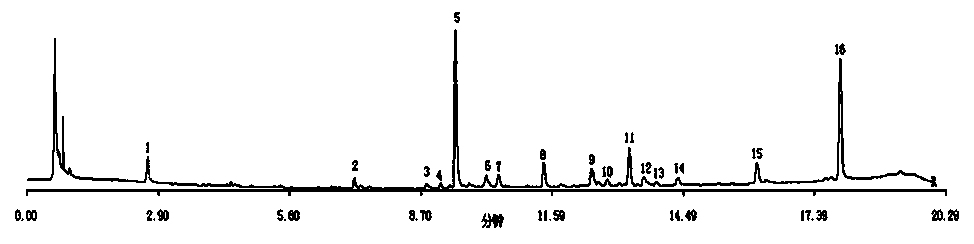
Detection method of liquorice formula granules
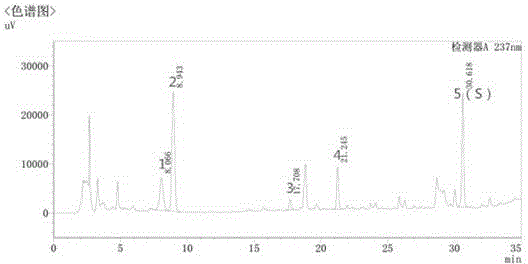
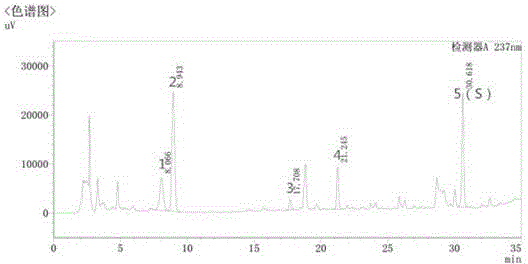
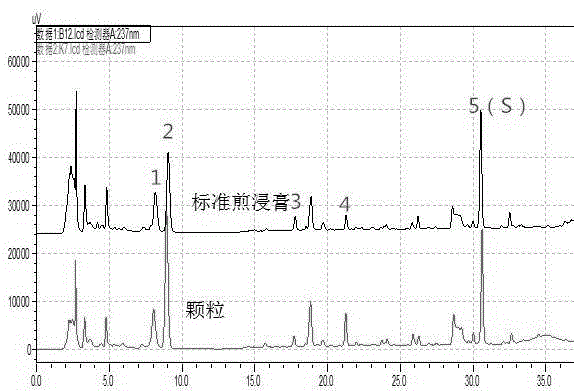
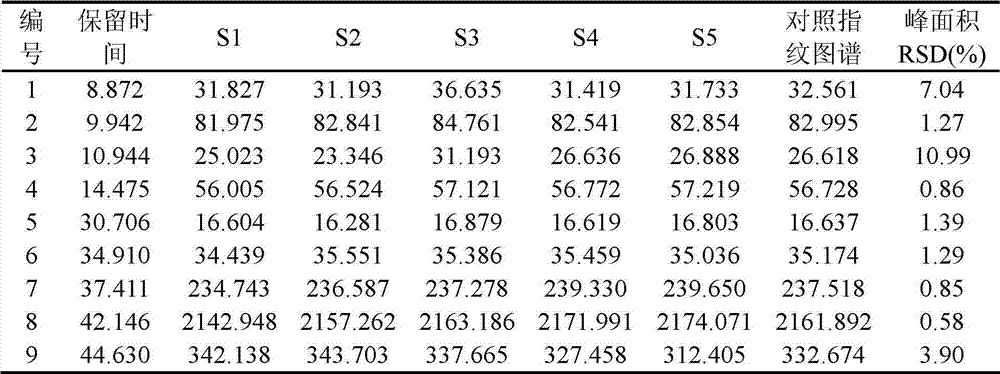
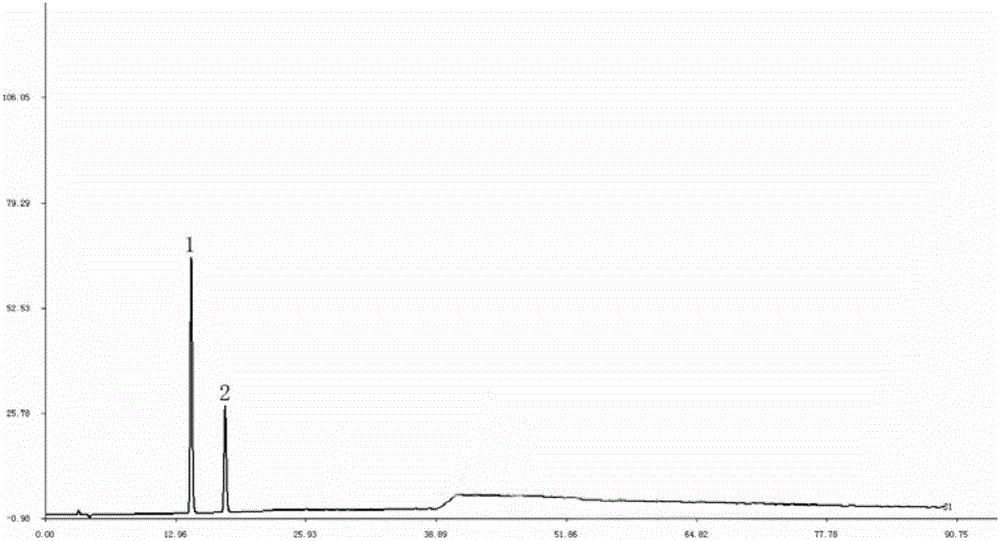
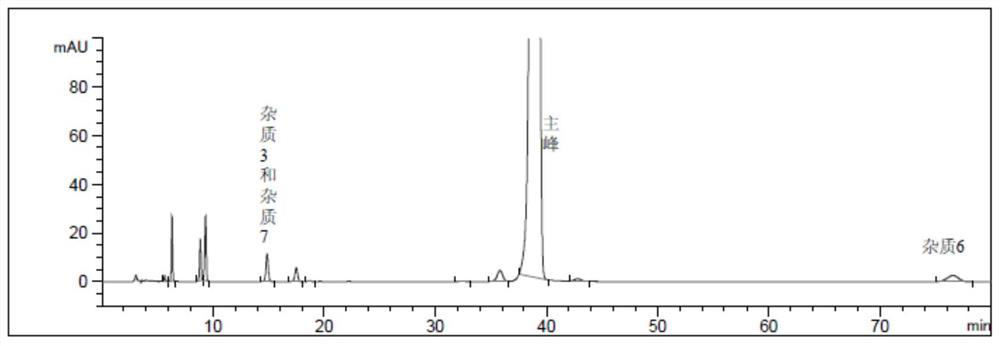
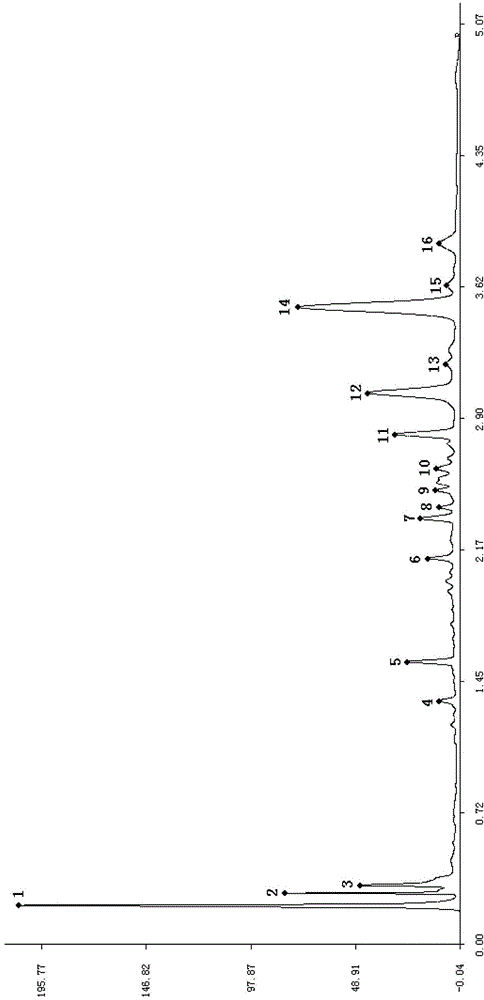
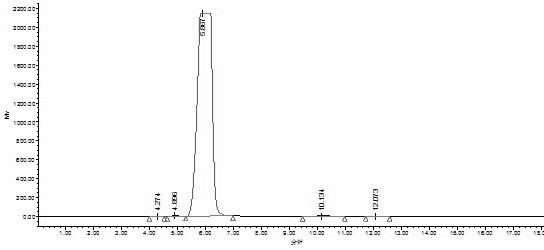
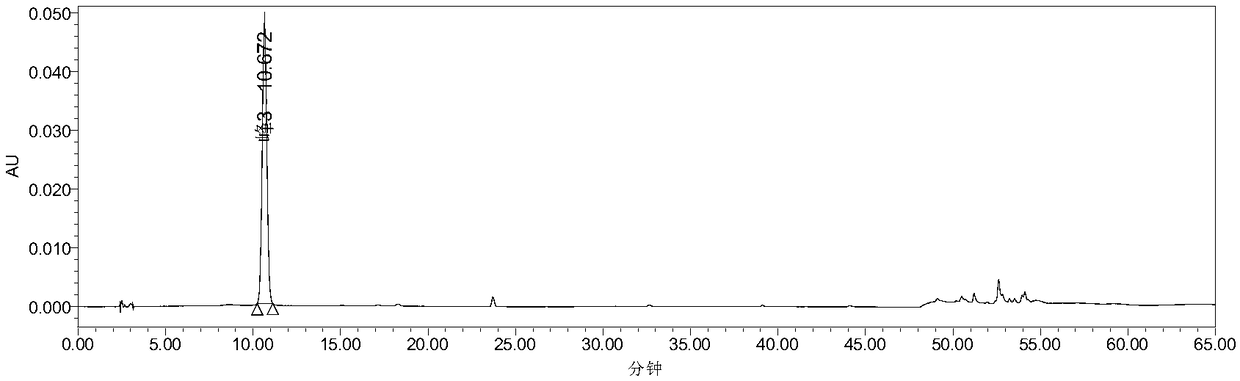
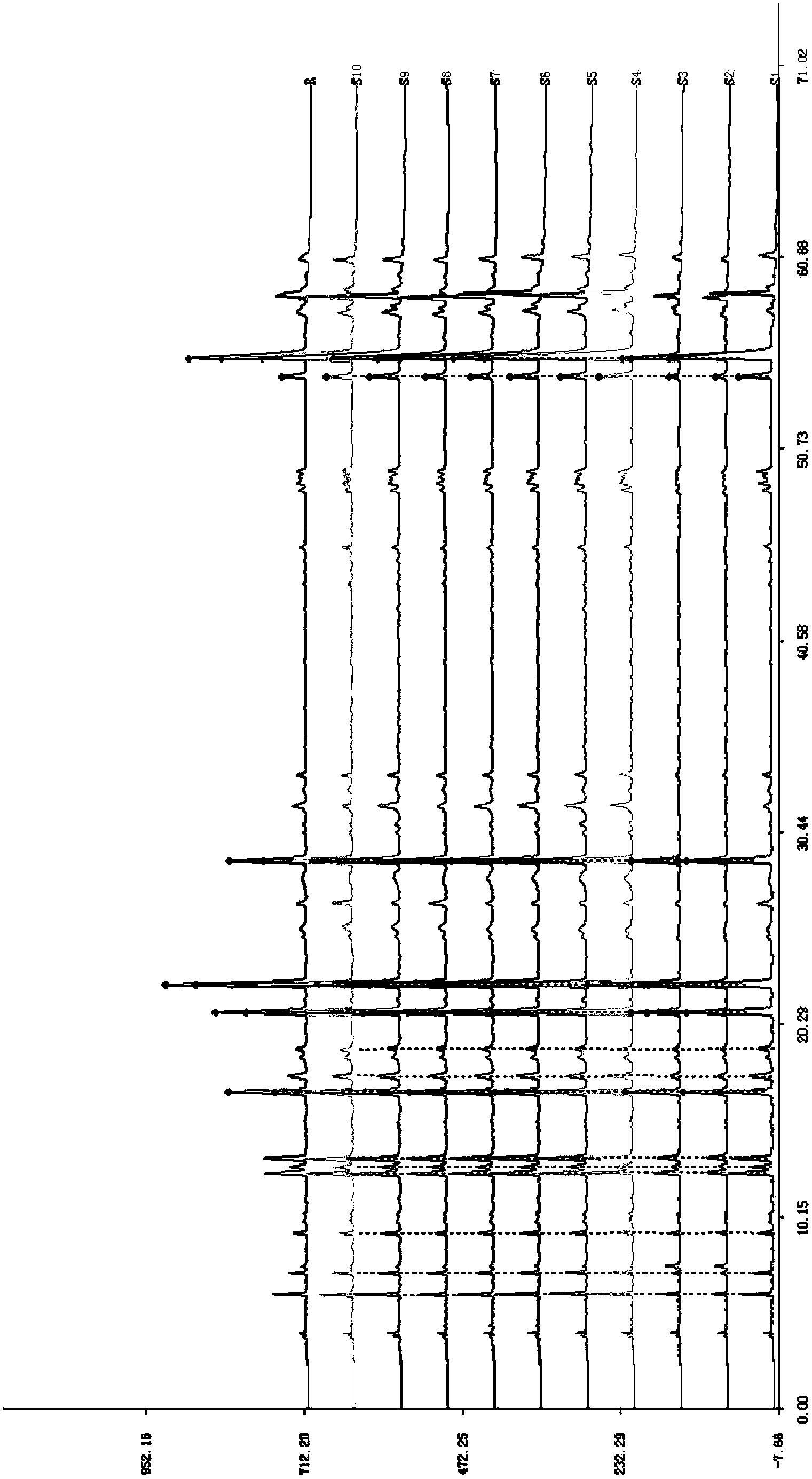
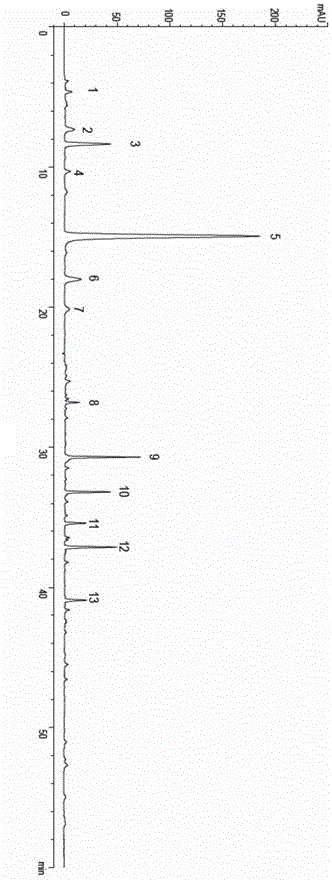
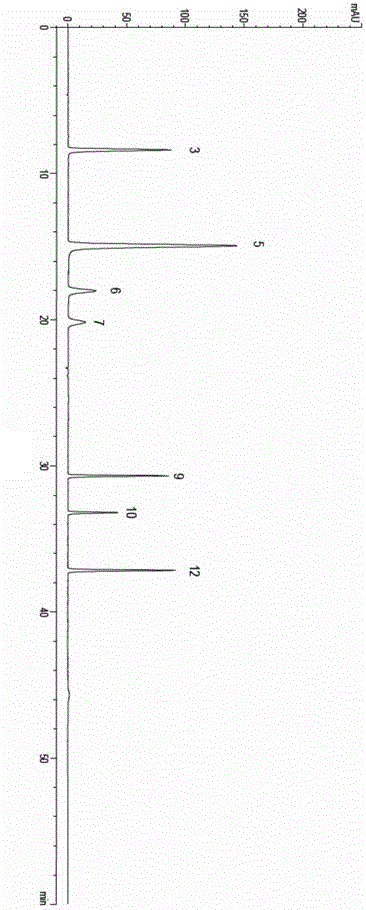
The invention discloses a quality control method of liquorice formula granules, belonging to the technical field of analysis of traditional Chinese medicines. The method is used for identifying the liquorice formula granules by adopting a high performance liquid characteristic chromatography, and comprises the following steps: (a) preparing a reference substance solution; (b) preparing a test solution; (c) detecting by adopting the high performance liquid chromatography to obtain the characteristic chromatogram of the liquorice formula granules. According to the established high performance liquid characteristic chromatogram of the liquorice formula granules, five characteristic peaks can be obtained, and as shown in the figure, the peak marked by No. 5 is a reference peak, and the retention time is taken as the following measured values: 8.066 minutes (peak 1), 8.943 minutes (peak 2), 17.708 minutes (peak 3), 21.245 minutes (peak 4) and 30.618 minutes (peak 5) (S). Every gram of liquorice formula granules is equivalent to 10-20g of crude drug. The method has the characteristics of being high in specificity and accuracy; the detection method is scientific and advanced and has operability. The characteristic chromatogram technology is applied to the quality control of the traditional Chinese medicine formula granules, so that the product quality is effectively controlled.
Owner:BEIJING KANGRENTANG PHARMA
Method for establishing Tripterygium wilfordii fingerprint chromatography and standard fingerprint chromatography thereof
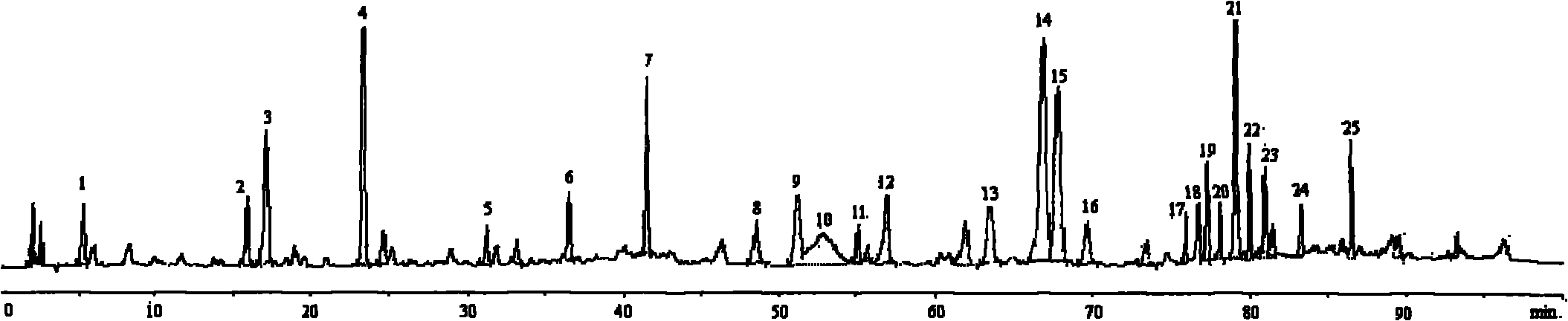
InactiveCN101642481AEasy to manufactureChromatographic conditions are easyAntibacterial agentsComponent separationHplc fingerprintRepeatability
The invention provides a method for establishing high performance liquid chromatography (HPLC) fingerprint chromatography of Tripterygium wilfordii and a standard fingerprint chromatography thereof, and the method comprises the steps of preparing test solution, selecting chromatographic conditions, manufacturing HPLC fingerprint chromatography, determining the standard fingerprint chromatography and the like. By comparing the HPLC Tripterygium wilfordii fingerprint chromatography in 17 batches to determine 24 common characteristic peaks, the common characteristic peaks form the fingerprint characteristics of the Tripterygium wilfordii which can be taken as the standard fingerprint chromatography of the Tripterygium wilfordii. The invention has the advantages of simple method, good repeatability, a lot of characteristic peaks, accuracy and reliability and the like. The established standard fingerprint chromatography can effectively represent quality of the Tripterygium wilfordii.
Owner:BIOCHEM ENG COLLEGE OF BEIJING UNION UNIV
Method for establishing rhizoma bletillae high performance liquid chromatography (HPLC) fingerprint spectrum and standard fingerprint spectrum thereof
InactiveCN103344717AEasy to manufactureChromatographic conditions are easyComponent separationHplc fingerprintRetention time
The invention provides a method for establishing a rhizoma bletillae high performance liquid chromatography (HPLC) fingerprint spectrum, as well as a rhizoma bletillae standard fingerprint spectrum obtained by using the method. According to the scheme, the method comprises the following steps: (1) preparing a test solution; and (2) performing HPLC chromatographic analysis. The rhizoma bletillae HPLC standard fingerprint spectrum has 13 chromatographic peaks which have the respective retention time of 2.024 minutes, 5.928 minutes, 10.765 minutes, 16.133 minutes, 17.279 minutes, 20.999 minutes, 23.232 minutes, 24.678 minutes, 25.924 minutes, 28.287 minutes, 30.366 minutes, 32.529 minutes and 34.291 minutes. According to the method, the test solution is easy and convenient to prepare, the chromatographic conditions are easily realized, the method is high in stability and reproducibility, the quality of rhizoma bletillae can be effectively controlled, and the method provides reference for quality control of rhizoma bletillae.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
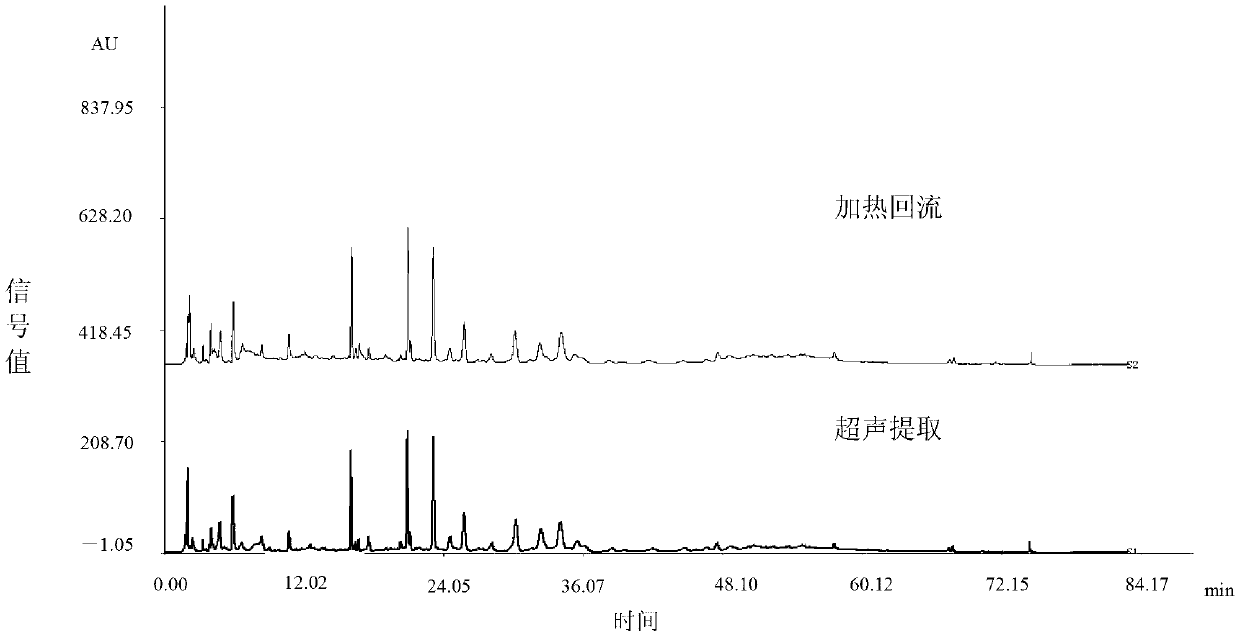
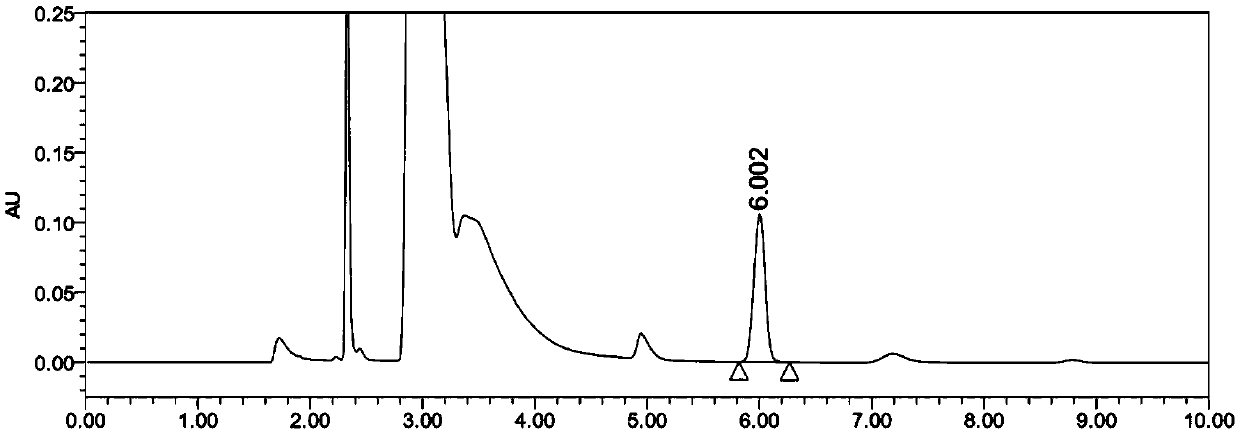
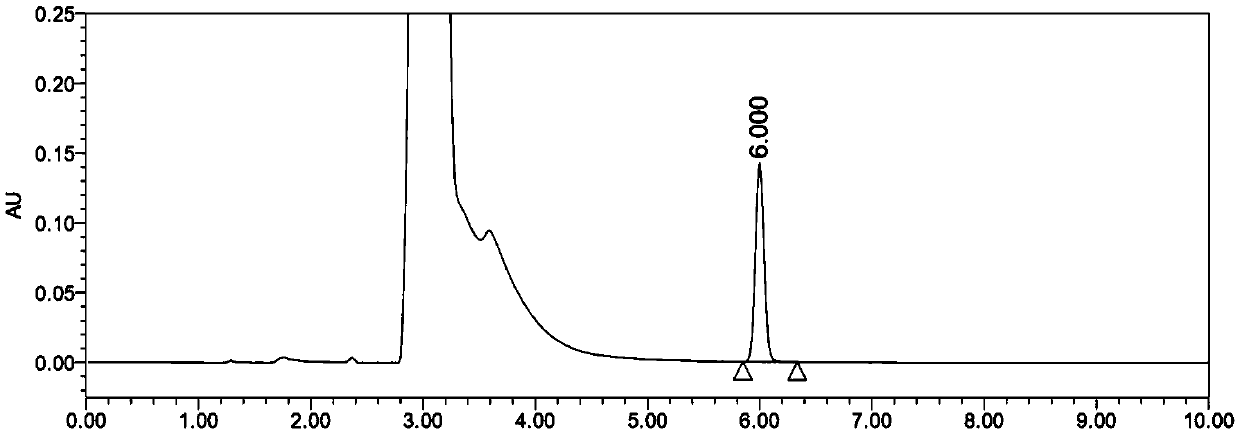
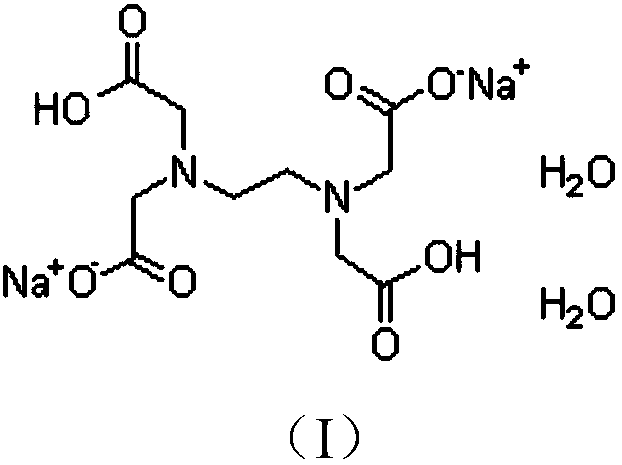
Content determination method of edetate disodium in acetylcysteine liquid preparation
ActiveCN110794045AEliminate distractionsImprove stabilityComponent separationDisodium EdetateCyclodextrin Derivatives
The invention belongs to the field of medicine detection, and discloses a content determination method of edetate disodium in acetylcysteine liquid preparation. In the method, HPLC is used, a test solution is prepared from the acetylcysteine liquid preparation, cyclodextrin and / or cyclodextrin derivatives, Fe3+ and a mobile phase, amino silane bonded silica gel is used as a chromatographic columnof a filler, a mixed solvent of ion pair buffer solution and an organic phase is used as the mobile phase, and thus, content of the edetate disodium in the test solution can be determined. The methodis simple to operate and excellent in repeatability, can accurately determine content of the edetate disodium in the acetylcysteine liquid preparation, and consequently can control product quality ofthe edetate disodium in acetylcysteine liquid preparation better.
Owner:上海聚可医药科技有限公司
Method for establishing HPLC (high performance liquid chromatography) finger-print of ophiopogon japonicus and standard finger-print thereof
InactiveCN102119997AEasy to manufactureChromatographic conditions are easyNervous disorderComponent separationHplc fingerprintTest article
The invention provides a method for establishing an HPLC (high performance liquid chromatography) finger-print of ophiopogon japonicus and a standard finger-print thereof. The method comprises the following steps of: preparing a test article solution; preparing a reference substance solution; selecting chromatographic conditions; and measuring with a high performance liquid chromatograph to obtain the finger-print of ophiopogon japonicus. 16 batches of HPLC finger-prints of ophiopogon japonicus are compared to determine the common finger-print characteristics of ophiopogon japonicus, thereby obtaining the standard finger-print. The method provided by the invention is simple, convenient, accurate and reliable, and has the characteristics of good reproducibility, multiple characteristic peaks and the like. The established standard finger-print can effectively show the quality of ophiopogon japonicus.
Owner:刘江 +1
Construction method of UPLC fingerprint of medicinal raw ginger and standard fingerprint thereof
InactiveCN104165933AEasy to prepareChromatographic conditions are easyComponent separationGradient elutionColumn temperature
The invention provides a construction method of ultrahigh performance liquid chromatography (UPLC) fingerprint of medicinal raw ginger, and belongs to the technical field of pharmaceutical analysis. The method is characterized by comprising the following steps: (1) precisely weighing about 1.25 grams of raw ginger, placing the raw ginger into a volumetric flask with a volume of 10 mL, adding methanol until the methanol reaches the scale, subjecting the volumetric flask to a supersonic treatment for 25 minutes, cooling, weighing, adding methanol to compensate the weight loss, shaking, filtering with a filter membrane with a pore size of 0.22 [mu]m, saving the filtrate for later use; (2) fingerprint preparation: adopting an ultrahigh performance liquid chromatography (UPLC) system, wherein the chromatographic column is a UPLCBEHC18 chromatographic column (2.1mm*100mm, 1.7[mu]m); acetonitrile (A)-water solution (B) is the mobile phase, gradient elution is adopted, the detection wavelength is 280 nm, the flow speed is 0.35 mL.min<-1>, and the column temperature is 35 DEG C, carrying out analysis so as to obtain UPLC fingerprint of medicinal raw ginger; (3) determination of standard fingerprint: establishing UPLC fingerprints of 17 batches of medicinal raw gingers from different production areas, and determining 16 common characteristic peaks, wherein the fingerprint characteristic of medicinal raw ginger is composed of the 16 common characteristic peaks, and can be used as the standard fingerprint of medicinal raw ginger.
Owner:韩燕全
Chinese angelica standard decoction fingerprint spectrum, characteristic spectrum establishment method and content determination method
InactiveCN110068628AGood fingerprint similarityStable and reliable quality standardsComponent separationFingerprintTest solution
The invention discloses a Chinese angelica standard decoction fingerprint spectrum, a characteristic spectrum establishment method and a content determination method. The establishment method for thefingerprint spectrum comprises the following steps of 1) preparing a test solution; 2) carrying out chromatographic condition and system suitability test; and 3) injecting the test solution into a liquid chromatograph, and measuring to obtain a fingerprint spectrum. An ultra-high performance liquid chromatography fingerprint spectrum of the Chinese angelica standard decoction is researched, so that a better Chinese angelica standard decoction fingerprint spectrum is obtained, and a novel technical method is provided for controlling the quality of the preparation.
Owner:SINOPHARM GRP DEZHONG (FOSHAN) PHARM CO LTD
Method for establishing HPLC fingerprint of Liubanhong garlic enzymatic hydrolysis product
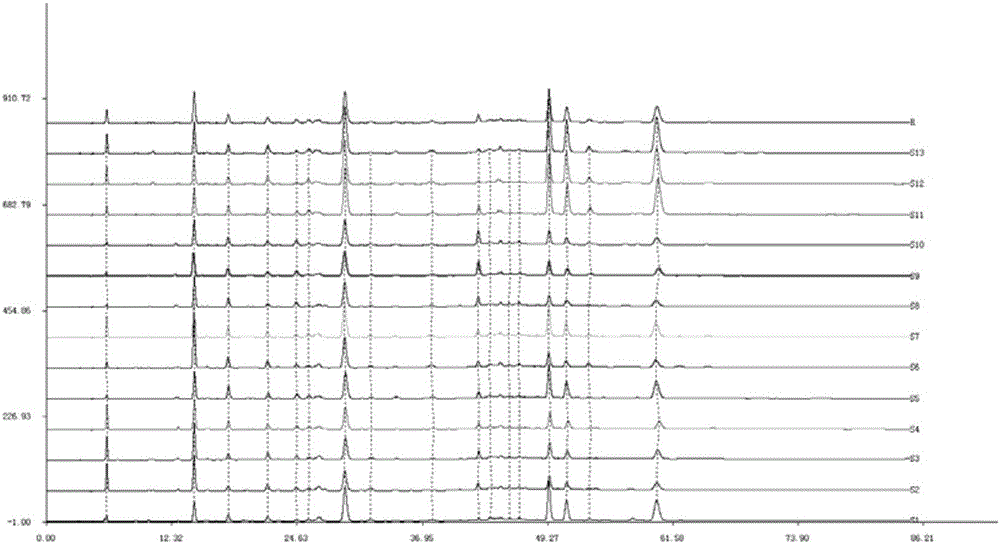
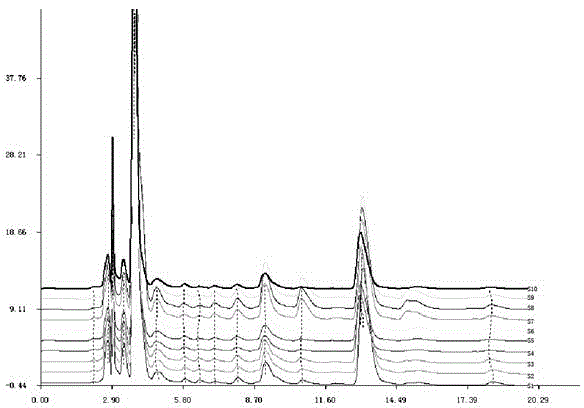
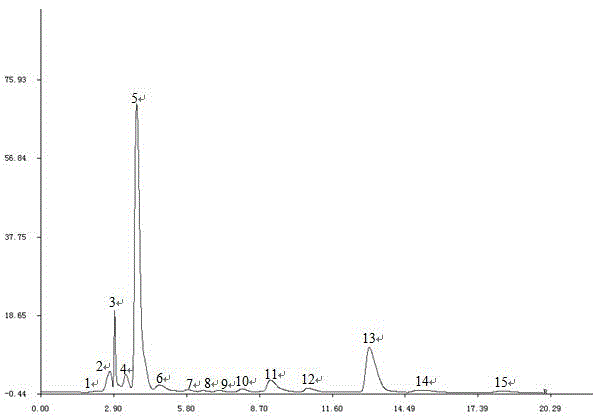
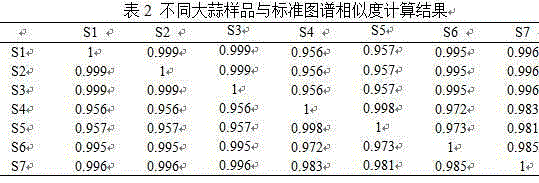
The invention discloses a method for establishing the HPLC fingerprint of a Liubanhong garlic enzymatic hydrolysis product. The method concretely comprises the following steps: carrying out solid phase extraction on bulbs of Liubanhong garlic which is a special kind in Baodi to prepare a test article solution, and carrying out high performance liquid chromatography analysis detection under certain conditions to obtain the HPLC fingerprint of the Liubanhong garlic enzymatic hydrolysis product. A standard fingerprint is obtained by determining common characteristic peaks HPLC fingerprint analysis of 10 batches of samples. The standard fingerprint can provide reliable bases for discrimination of the kind and the producing area of the Liubanhong garlic and production quality control. The method has the advantages of convenient operation, high stability, strong specificity, good reappearance, many characteristic peaks, high analysis efficiency, true and false and producing area discrimination of the Liubanhong garlic, and comprehensive, accurate and effective evaluation of the internal quality of the Liubanhong garlic.
Owner:TIANJIN NORMAL UNIVERSITY
Method for establishing coltsfoot flower medicine fingerprint and standard fingerprint thereof
InactiveCN102520102AEasy to manufactureChromatographic conditions are easyComponent separationHplc fingerprintMedicinal herbs
The invention provides a method for establishing a coltsfoot flower medicine HPLC (High Performance Liquid Chromatography) fingerprint and a standard fingerprint thereof. The method comprises the following steps of: taking coltsfoot flower medicine powder, adding ethanol, carrying out ultrasonic extraction, filtering supernate by using a micro-pore filter film, and taking the filtrate as a sample solution; then, carrying out high-performance liquid chromatography, wherein a chromatographic column is a Diamonsil-C18 column (5 mum, 250*4.6 mm); taking 0.1% of phosphoric acid-acetonitrile as a mobile phase, and adopting a gradient elution way; detecting the wavelength of 240 nm and the column temperature of 30 DEG C; introducing the sample by absorbing the sample solution to obtain the coltsfoot flower medicine HPLC fingerprint; and comparing 10 batches of coltsfoot flower medicine HPLC fingerprints and determining common fingerprint characteristics to obtain the standard fingerprint. The quality of coltsfoot flower medicine can be effectively monitored by utilizing the standard fingerprint, so that stability, uniformity and controllability of the coltsfoot flower medicine can be kept.
Owner:TAISHAN MEDICAL UNIV
Radix salvia miltiorrhiza fingerprint establishment method and standard fingerprint
InactiveCN102928538ASignificant advantagesSignificant useComponent separationBiotechnologySalvia miltiorrhiza
The invention relates to a traditional Chinese medicine (TCM) fingerprint establishment method, in particular to a radix salvia miltiorrhiza high-performance liquid chromatograph (HPLC) fingerprint establishment method and a radix salvia miltiorrhiza standard fingerprint obtained by adopting the method, provides a TCM fingerprint establishment method and a standard fingerprint, and aims to solve the problem that TCM substance identification and composition analysis can not effectively reflect and control the total body mass of TCM at present. The method comprises the following steps: firstly, preparation of a test solution; and secondly, high-performance liquid chromatograph analysis. The radix salvia miltiorrhiza fingerprint can be obtained by adopting the method. The method is used in the technical field of medicine analysis.
Owner:哈药集团中药二厂
Establishment method of hplc fingerprint of Zhuang medicinal material Diangui Ainaxiang
ActiveCN104458993BLarge amount of informationRealize evaluationComponent separationHplc fingerprintChemical composition
The invention discloses a method for establishing an HPLC fingerprint spectrum of a Zhuang medicinal material Blumea riparia (Bl.) DC. By adopting a high-performance liquid chromatography, and using main ingredients such as protocatechuic acid and protocatechuic aldehyde of Blumea riparia (Bl.) DC as reference substances, a fingerprint spectrum of a common pattern of the Zhuang medicine Blumea riparia (Bl.) DC medicinal material is obtained. The fingerprint spectrum has 13 chromatographic peaks, can fully reflect the chemical compositions of the Blumea riparia (Bl.) DC medicinal material, and is rich in information amount, the method has good reproducibility, a more powerful theoretical basis is provided for controlling the quality of the medicinal material and identifying the advantages and disadvantages of the medicinal material, the quality control and the level of true and false identification of the Zhuang medicine Blumea riparia (Bl.) DC medicinal material are improved, and the authenticity and the advantages and disadvantages of the Blumea riparia (Bl.) DC can be quickly and accurately identified.
Owner:广西万寿堂药业有限公司
Establishment method of HPLC (High Performance Liquid Chromatography) fingerprint spectrum of allinase inactivated extract of six-peal red garlics
InactiveCN105158369AEasy to handleLess impuritiesComponent separationHplc fingerprintSolid phase extraction
The invention discloses an establishment method of an HPLC (High Performance Liquid Chromatography) fingerprint spectrum of an allinase inactivated extract of six-peal red garlics. The establishment method comprises the following specific steps of preparing six-peal red bulbs which belong to a peculiar variety in Baodi County into a test product solution by a solid-phase extraction step and the like; performing HPLC detection under certain conditions to obtain the HPLC fingerprint spectrum of the allinase inactivated extract of the six-peal red garlics; analyzing the HPLC fingerprint spectrums of ten batches of samples to determine a common characteristic peak and obtaining a standard fingerprint spectrum which can provide a reliable basis for the identification of varieties and production places of the six-peal red garlics and control over the production quality. The establishment method disclosed by the invention has the advantages of convenience in operation, high stability, high specificity, good reproducibility, more characteristic peaks and high analytic efficiency; by using the establishment method disclosed by the invention, the authenticity and production place of the six-peal red garlics can be identified, and the internal quality of the six-peal red garlics can be evaluated comprehensively, accurately and effectively.
Owner:TIANJIN NORMAL UNIVERSITY
Establishment method for high performance liquid chromatography fingerprint of Rubia cordifolia root formula granule and standard fingerprint thereof
ActiveCN104133028AEffective quality controlEasy to manufactureComponent separationRetention timeQuality control
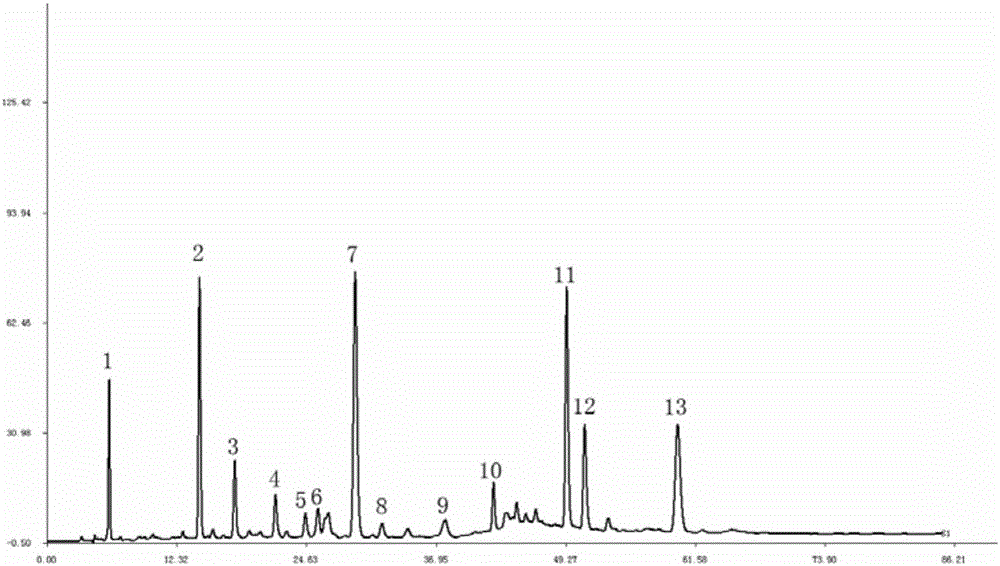
The invention provides an establishment method for a high performance liquid chromatography (HPLC) fingerprint of a Rubia cordifolia root formula granule, and a Rubia cordifolia root formula granule standard fingerprint obtained thereby. The establishment method provided by the invention includes the steps of: (1) preparation of a test solution; and (2) high performance liquid chromatography analysis. The high performance liquid chromatography standard fingerprint of the Rubia cordifolia root formula granule aqueous solution obtained by the method has a total of 11 chromatographic peaks, the retention time of which is 6.072min, 6.559min, 8.087min, 9.170min, 12.118min, 20.988min, 29.251min, 32.211min, 33.189min, 38.430min and 45.431min respectively. The HPLC standard fingerprint of the Rubia cordifolia root formula granule fat-soluble components has a total of 9 chromatographic peaks, the retention times of which is 12.156min, 12.832min, 14.552min, 22.615min, 23.291min, 25.412min, 27.356min, 34.383min and 37.076min respectively. According to the invention, the test solution is simple to prepare, the chromatographic conditions are easy to realize, and the method has good stability and reproducibility, can effectively control the quality of the Rubia cordifolia root formula granule, and provides reference for quality control of the Rubia cordifolia root formula granule.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE +1
Determination method of alanyl-glutamine related substances
PendingCN113933403AChromatographic conditions are simpleEasy to operateComponent separationChromatographic columnOctadecane
Owner:SUZHOU KELUN PHARMA RES CO LTD
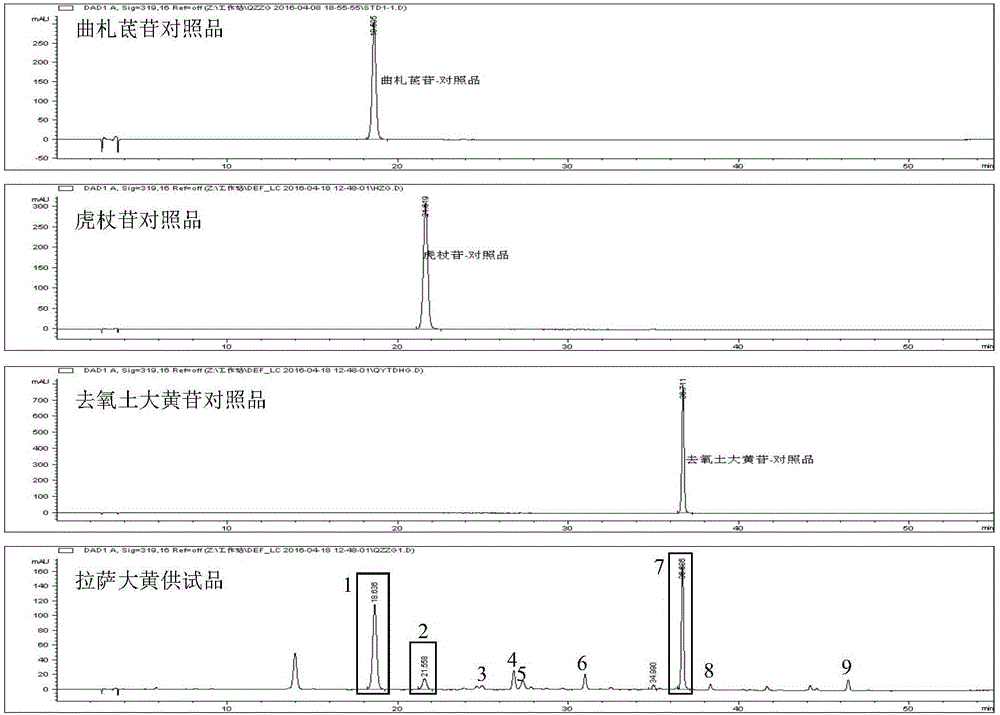
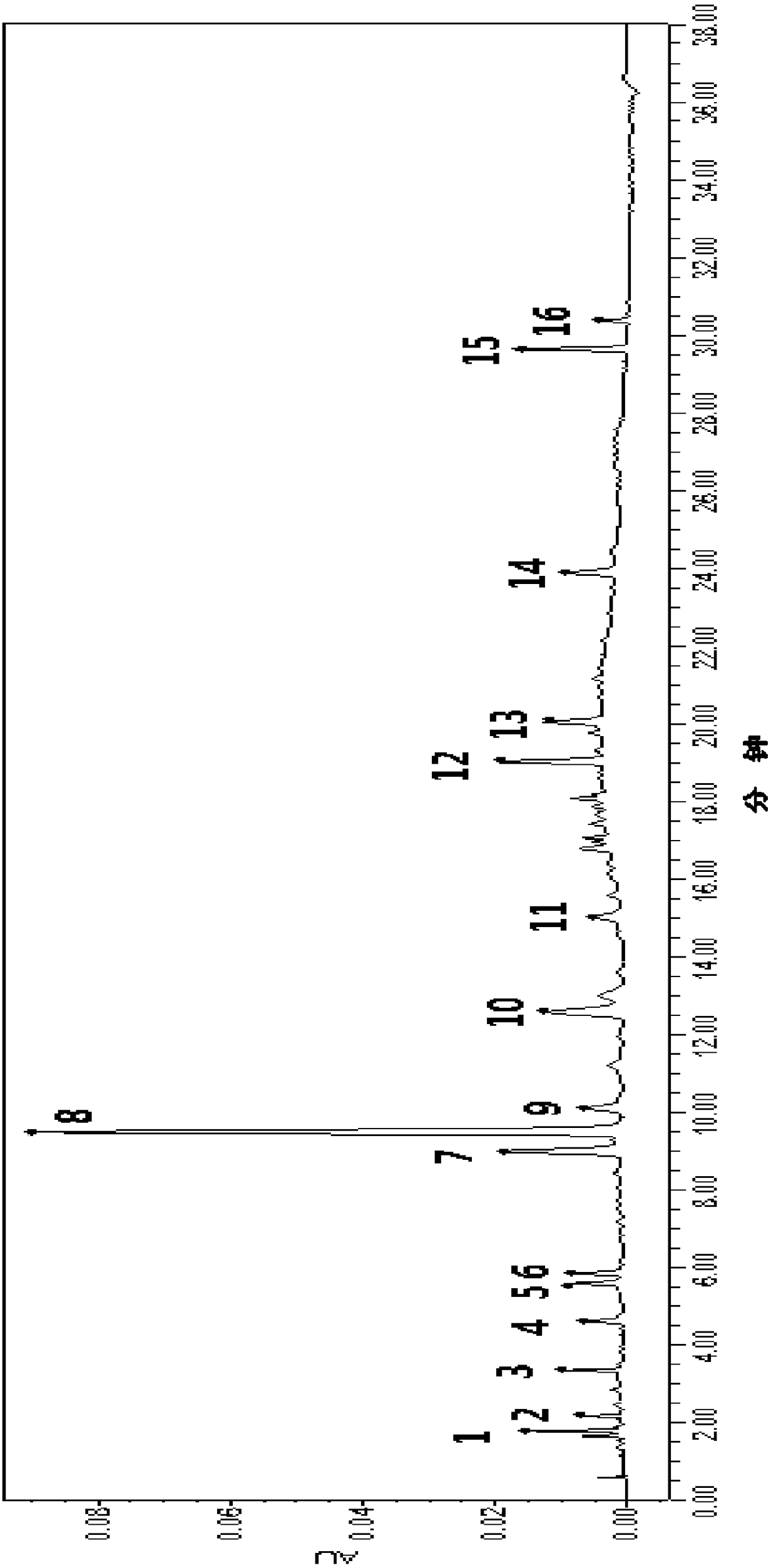
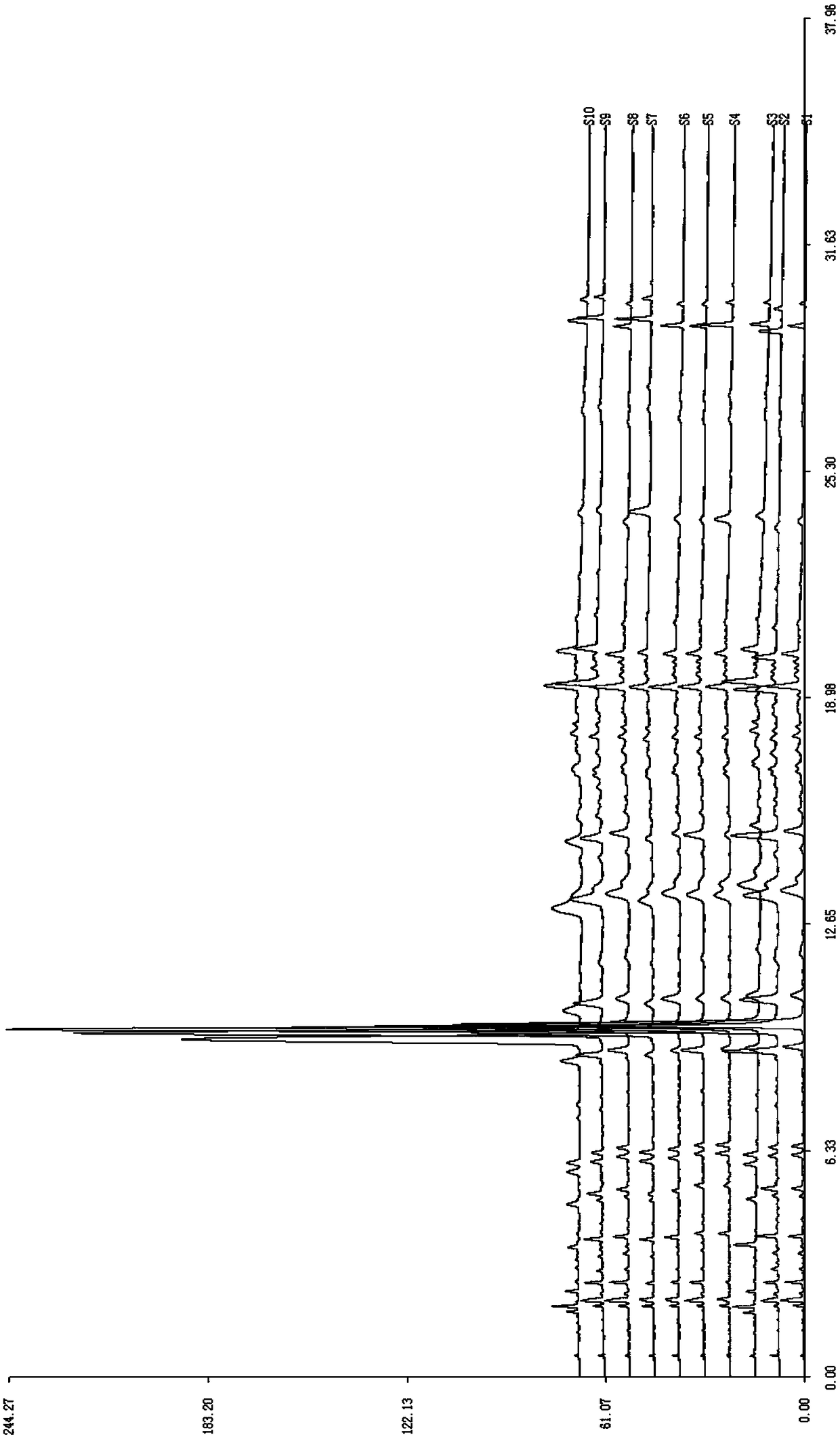
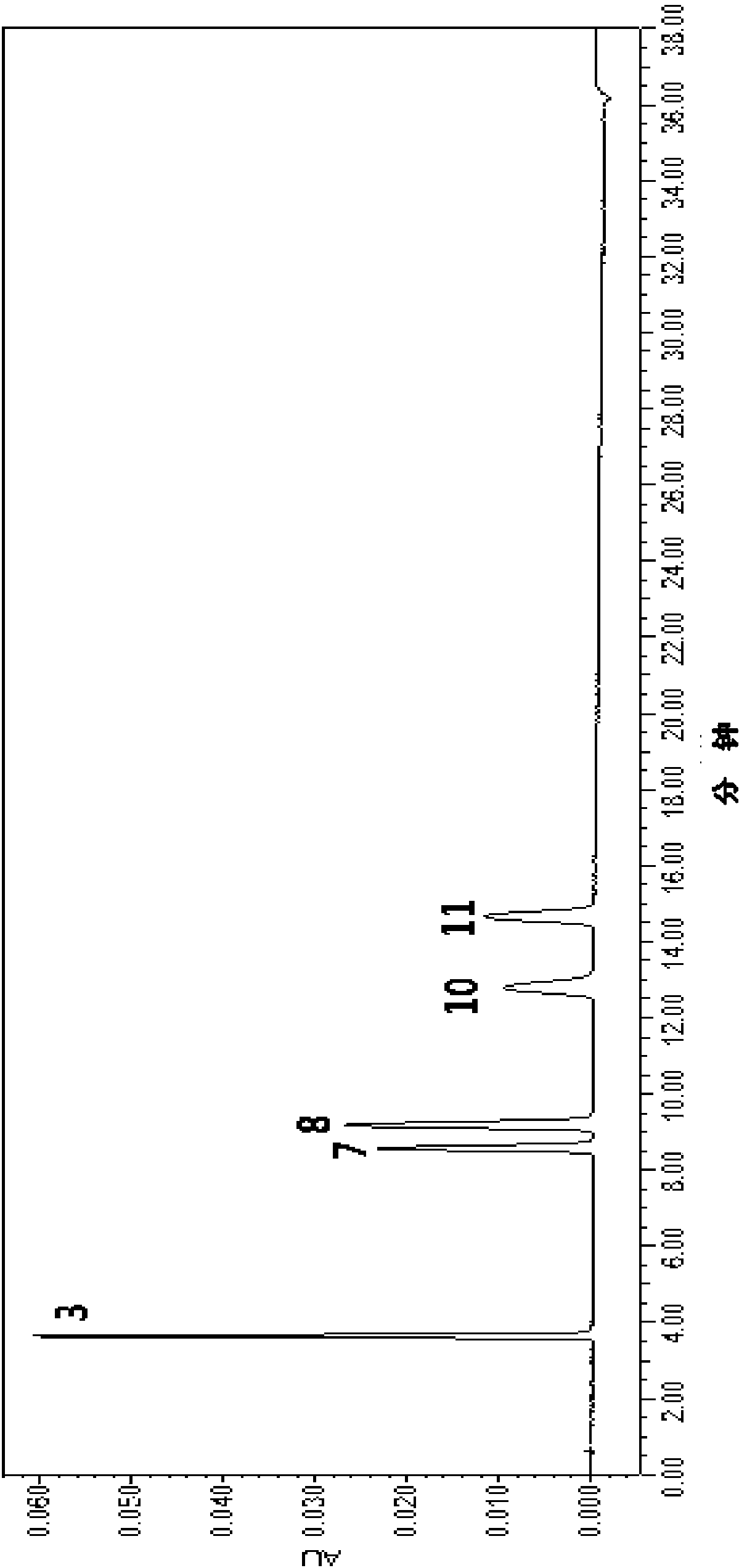
Construction method of rheum lhasaense fingerprint spectrum and standard fingerprint spectrum of rheum lhasaense
The invention provides a construction method of a rheum lhasaense fingerprint spectrum and a standard fingerprint spectrum of rheum lhasaense. High performance liquid chromatograph is conducted on a rheum lhasaense sample through a chromatographic column taking octadecylsilane chemically bonded silica as packing, the fingerprint spectrum is constructed by taking piceatannol 3'-O-glucoside as a reference peak, and the fingerprint spectrum is evaluated on this basis. The obtained rheum lhasaense fingerprint spectrum can comprehensively, systematically and characteristically reflect composition ingredients in rheum lhasaense, comprehensively and effectively evaluate the quality of rheum lhasaense and objectively reflect authenticity and merits of a product and is beneficial for product quality monitoring. The method is easy and convenient to operate, stable, high in precision and good in reproducibility.
Owner:KPC PHARM INC
Method for constructing UPLC fingerprint of Callicarpa nudiflora medicinal material, and standard fingerprint
ActiveCN108663440AAddress effectivenessSolve quality problemsComponent separationFingerprintOnline analysis
The invention provides a method for constructing the UPLC fingerprint of a Callicarpa nudiflora medicinal material, and a standard fingerprint of the Callicarpa nudiflora medicinal material, obtainedthrough the method. The method for constructing the UPLC fingerprint of the Callicarpa nudiflora medicinal material comprises the following steps: preparing a sample solution, and carrying out ultra performance liquid chromatography. The method has the characteristics of simplicity, easiness in realization of chromatography conditions, high precision, and goo stability and good reproducibility ofan analysis result; and compared with other test analysis methods in the prior art, the method in the invention has the advantages of shortening of the online analysis time and saving of the materialcost and the time cost.
Owner:HAINAN JIUZHITANG PHARMACY +1
Construction method of UPLC fingerprint of sweet clover medicinal material and standard fingerprint thereof
ActiveCN105699581AAddress effectivenessSolve quality problemsComponent separationTest sampleTest analysis
The invention provides a method for constructing the UPLC fingerprint of Medicinal Rhizome and the standard fingerprint of Medicinal Rhododendron obtained by the method. The method for constructing the UPLC fingerprint of Medicinal Rhizome according to the present invention includes the steps of preparing the test sample solution and the step of ultra-high performance liquid chromatography analysis. According to the method of the present invention, the method is simple, the chromatographic analysis conditions are easy to realize, the precision is high, and the stability and reproducibility of the analysis results are better. Compared with other test and analysis methods in the prior art, the online analysis time is shortened and the Material cost and time cost.
Owner:STAIDSON (BEIJING) BIOPHARMACEUTICALS CO LTD +1
Method for analyzing the purity of N-p-aminobenzoyl-L-glutamic acid through liquid chromatography
InactiveCN102012408AImprove stabilityGood reproducibilityComponent separationAnalytical techniqueMedical treatment
The invention relates to an analysis technology of pharmaceutical intermediates, in particular to a method for analyzing the purity of N-p-aminobenzoyl-L-glutamic acid through chromatography. No method for analyzing the purity of an N-p-aminobenzoyl-L-glutamic acid sample exits in the prior art so that the purity and the quality of the N-p-aminobenzoyl-L-glutamic acid sample can not be analyzed and monitored, thus the quality of a folic acid synthesized by using the N-p-aminobenzoyl-L-glutamic acid as a raw material can not be ensured, and medical effect can not be ensured. In the method of the invention, the purity of the N-p-aminobenzoyl-L-glutamic acid sample is analyzed with an internal standard curve method by adopting a common C18 column matching with an ultraviolet detector, taking an H3PO4-KH2PO4 buffer solution-methanol system as a mobile phase and adopting a sulfanilic acid as an internal standard substance, wherein the flow velocity of the mobile phase is set as 0.3 milliliter / minute. The method has the advantages of easily realized analysis conditions, high stability of the sample, good repeatability of an analysis result and high accuracy of the analysis result.
Owner:SHANGHAI NORMAL UNIVERSITY
Method for constructing fingerprint of Yangweishu granules
ActiveCN109030641AQuality assuranceSimple and fast operationComponent separationEvaluation systemAnalysis method
The invention discloses a method for constructing a fingerprint of Yangweishu granules. The method comprises the steps of: extracting the Yangweishu granules as atest solution, determining at least 10batches of the Yangweishu granules according to an optimized chromatographic condition to obtain a corresponding chromatogram, and introducing the chromatogram into the similarity evaluation system of the traditional Chinese medicine chromatographic fingerprint prescribed by the Chinese Pharmacopoeia Commission to obtain the fingerprint of the Yangweishu granules. The method for constructing thefingerprint of the Yangweishu granuleshas the characteristics of convenience and simplicity in method, good stability, high precision and good reproducibility, can fully reflect material information of a traditional Chinese medicine preparation, and provides a new analytical method for quality inspection and quality control of the Yangweishu Granules.
Owner:合肥华润神鹿药业有限公司
Establishment method of scorzonera fingerprint spectrum and scorzonera standard fingerprint spectrum
The invention provides an establishment method of a scorzonera fingerprint spectrum. The establishment method comprises the following specific steps: preparing a test solution by scorzonera, and carrying out fractionation detection under a certain condition by a high performance liquid chromatograph to obtain the scorzonera fingerprint spectrum. The invention further provides a scorzonera standard fingerprint spectrum obtained by the method. The standard fingerprint spectrum can provide reliable basis for scorzonera identification and internal mass control. The establishment method is simple and convenient to operate, high in stability, good in reproducibility and multiple in characteristic peaks. By utilizing the establishment method, the quality of scorzonera medicinal materials can be comprehensively and accurately evaluated.
Owner:JILIN UNIV
Determination method for fingerprint spectrum of Linaoxin Pian as well as standard fingerprint spectrum of Linaoxin Pian
ActiveCN109239250AEffectively characterize the content of ingredientsEffective massComponent separationHplc fingerprintMicropore Filter
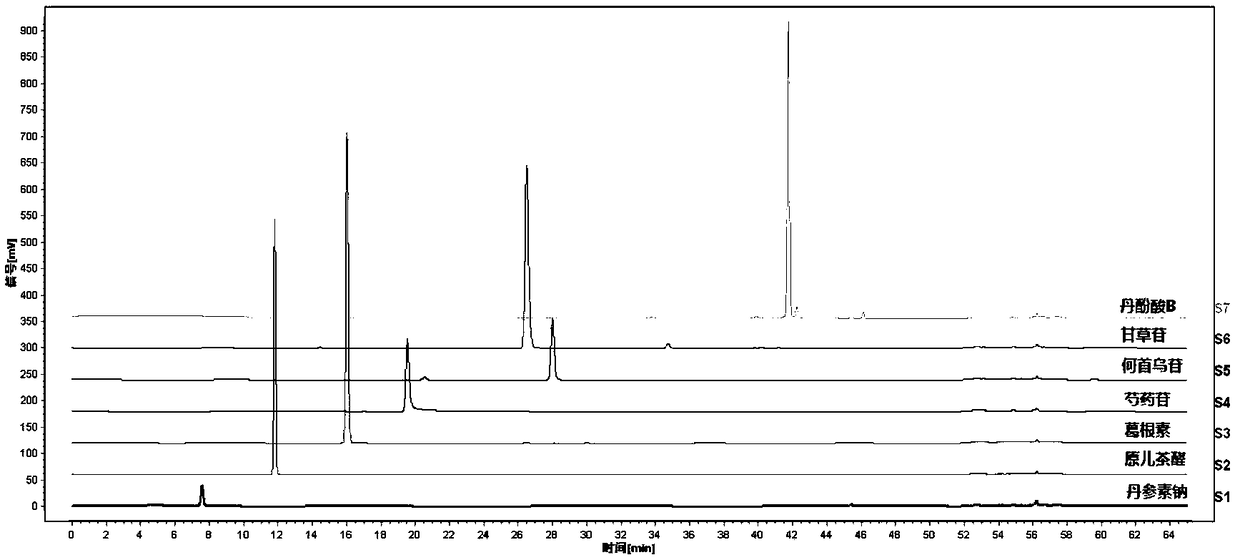
The invention discloses a determination method for an HPLC fingerprint spectrum of a Linaoxin Pian as well as a standard fingerprint spectrum of the Linaoxin Pian. The determination method comprises the following steps: (1) preparing reference solution, namely respectively preparing sodium tanshinol, protocatechualdehyde, puerarin, paeoniflorin, liquiritin, polygonum multiflorum glycoside and salvianolic acid B reference solution; (2) preparing test solution, namely taking the Linaoxin Pian, extracting, and filtering extract with a micropore filter membrane, so that the test solution is obtained; and (3) performing the determination method, namely performing determination by adopting high performance liquid chromatography, wherein chromatographic conditions comprise that a chromatographiccolumn takes octadecyl silane bonded silica gel as filler, gradient elution is adopted, a mobile phase A is 0.01-3% phosphoric acid aqueous solution, a mobile phase B is acetonitrile, flow rate is 0.3-1.5ml / min, column temperature is 20-45 DEG C, and detection wavelength is 230nm.
Owner:WUXI JIYU SHANHE PHARM CO LTD +1
Method for establishing HPLC fingerprint of dichloromethane part of root of costus speciosus (koening) smith as Yao medicinal material
ActiveCN110261525AAccurate distinctionEase of evaluationComponent separationHplc fingerprintComputer science
The invention discloses a method for establishing an HPLC fingerprint of a dichloromethane part of the root of costus speciosus (koening) smith as a Yao medicinal material. The method comprises the following steps: (1), preparing a test solution; (2), preparing a reference solution by using diosgenin as a standard product; (3), carrying out HPLC detection; to be specific, recording an HPLC map within 95 minutes; and (4), detecting a plurality of test products, carrying out matching of all chromatographic peaks by using "Traditional Chinese medicine chromatographic fingerprint similarity evaluation system" software and a multi-point calibration method, and generating a reference fingerprint R automatically as a reference fingerprint. According to the invention, with establishment of the fingerprint of the root of costus speciosus (koening) smith, the relative relationship of the components contained in the root of costus speciosus (koening) smith can be reflected comprehensively; the medicinal materials from different origin places can be distinguished effectively; and the quality can be evaluated and controlled well. Therefore, the development and utilization of the root of costus speciosus (koening) smith in Guangxi province can be promoted.
Owner:GUANGXI UNIV OF CHINESE MEDICINE
Establishment method of inducing callus cultured Allium sativum L. enzymatic hydrolysate HPLC fingerprint
The invention discloses an establishment method of inducing callus cultured Allium sativum L. enzymatic hydrolysate HPLC fingerprints. The establishment method of the inducing callus cultured Allium sativum L. enzymatic hydrolysate HPLC fingerprints specifically comprises the steps of preparing inducing callus cultured Allium sativum L. bulbs into solution for test products through steps such as solid-phase extraction, and performing high-performance chromatographic analysis and detection under certain conditions to obtain HPLC fingerprints of inducing callus cultured Allium sativum L. enzymatic hydrolysate. By analyzing the HPLC fingerprints of 10 batches of samples, characteristic common peaks are determined and standard fingerprints are obtained. The standard fingerprints can provide a reliable basis for variety identification and production quality control of inducing callus cultured Allium sativum L. The establishment method of the inducing callus cultured Allium sativum L. enzymatic hydrolysate HPLC fingerprints has the advantages that the operation is convenient, the stability is high, the specificity is strong, the repeatability is good, the characteristic peaks are more, the analysis efficiency is high, the truth identification of the inducing callus cultured Allium sativum L. can be realized by adopting the invention, and the interior product quality can be comprehensively, accurately and effectively evaluated.
Owner:TIANJIN NORMAL UNIVERSITY
Establishment method for variable-wavelength fingerprint spectrum of Chinese pulsatilla root decoction granules and standard fingerprint spectrum thereof
InactiveCN103389343AComprehensive responseEnhanced visual comparisonComponent separationUltraviolet lightsGradient elution
The invention discloses an establishment method for a variable-wavelength fingerprint spectrum of Chinese pulsatilla root decoction granules. The method comprises the following steps of (1) preparing reference substance solutions: preparing reference substance solutions of berberine hydrochloride, palmatine hydrochloride, coptisine, jatrorrhizine hydrochloride, aesculin, aesculetin and pulchinenoside anemoside B4 respectively; (2) preparing a test sample solution: weighing the Chinese pulsatilla root decoction granules, extracting the Chinese pulsatilla root decoction granules and filtering an extracted solution by using a microporous membrane to obtain the test sample solution; (3) obtaining the fingerprint spectrum by determining with high performance liquid chromatography, wherein chromatographic conditions are as follows: octadecylsilane chemically bonded silica is used as a filler by a chromatographic column; gradient elution is adopted; variable wavelengths are detected by ultraviolet light wavelengths; and (4) evaluating similarity: evaluating the fingerprint spectrum of the test sample by "Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese medicine (Edition 2004A)". The method is simple, stable, high in precision and good in repeatability, and can identify the authenticity and merits of products accurately.
Owner:DIHON PHARMA GROUP +1
Establishment method of Aquilaria sinensis leaf high performance liquid chromatography (HPLC) fingerprint spectrum
InactiveCN104569236AEasy to separateStrong characteristicComponent separationHplc fingerprintTest article
The invention relates to the field of the quality control technology of traditional Chinese medicinal materials and in particular discloses an establishment method of an Aquilaria sinensis leaf high performance liquid chromatography (HPLC) fingerprint spectrum. The establishment method comprises the steps of preparing reference solution, preparing test article solution, determining the chromatographic conditions of the fingerprint spectrum, and determining a standard fingerprint spectrum; the establishment method is characterized in that the chromatographic conditions of the fingerprint spectrum are as follows: a C18 chromatographic column is adopted, acetonitrile-0.1-0.5% of phosphate buffer solution is used as mobile phase for gradient elution; an ultraviolet detector is used for detection and the detection wavelength is 300-360nm. The fingerprint spectrum has strong characteristic feature, main common peaks can achieve good baseline separation, a test article can be prepared simply and conveniently, the chromatographic conditions can be realized easily, and the method is simple and convenient, has good stability and good reproducibility and is suitable for the quality control of Aquilaria sinensis leaves.
Owner:DONGGUAN MATHEMATICAL ENG ACAD OF CHINESE MEDICINE GUANGZHOU UNIV OF CHINESE MEDICINE
Lumbar kidney cream fingerprint spectrum establishing method and Lumbar kidney reference fingerprint spectrum
The invention discloses a lumbar kidney cream fingerprint spectrum establishing method and a lumbar kidney reference fingerprint spectrum. The fingerprint spectrum establishing method comprises the steps that (1) a sample is pretreated, wherein volatile components in lumbar kidney cream are absorbed by ethyl acetate to serve as a test sample for gas phase identification, water is added into the remaining cream, and the mixture is used as a test sample for liquid phase identification after being subjected to methanol ultrasonic extraction and liquid immersion; (2) gas phase analysis is performed on the test sample for gas phase identification to obtain a lumbar kidney cream gas phase fingerprint spectrum; and (3) liquid phase analysis is performed on the test sample for liquid phase identification to obtain a lumbar kidney cream liquid phase fingerprint spectrum. Through research about the lumbar kidney cream fingerprint spectra, a good lumbar kidney cream fingerprint spectrum is obtained, and a new technical method is provided for controlling the quality of the preparation.
Owner:SINOPHARM GRP DEZHONG (FOSHAN) PHARM CO LTD
Method for establishing fingerprint of leech and standard fingerprint of leech
The invention provides a method for establishing a fingerprint of leech. The method comprises the following specific steps: preparing the leech into a test solution; and performing separation detection through a high performance liquid chromatograph under certain conditions to obtain the fingerprint of the leech. The invention also provides a standard fingerprint of the leech, which is obtained through the method. The fingerprint can provide a reliable basis for leech identification and internal quality control. The method is simple and convenient to operate, high in stability, high in repeatability and large in quantity of characteristic peaks, and is capable of comprehensively and accurately evaluating the quality of leech medicine.
Owner:LUNAN HOPE PHARM CO LTD
Method for establishing quantitative fingerprint spectrum of standard fructus evodiae decoction
PendingCN114705779AEasy to operateChromatographic conditions are easy to achieveComponent separationTraditional Chinese medicineTest solution
The invention discloses a method for establishing a quantitative fingerprint spectrum of a fructus evodiae standard decoction, and relates to the field of establishment of fingerprint spectrums.The method comprises the following steps that 1, a reference substance solution is prepared; (2) preparing a test solution; (3) carrying out ultra-high performance liquid chromatography analysis; and (4) establishing a quantitative fingerprint, introducing multiple batches of liquid chromatograms into a traditional Chinese medicine chromatographic fingerprint similarity evaluation system, matching common peaks, generating a control characteristic spectrum, selecting reference peaks, calculating the relative retention time of each characteristic peak, and determining the content of three components, namely limonin, evodiamine and rutaecarpin. And establishing a quantitative fingerprint spectrum of the fructus evodiae standard decoction. According to the method, the characteristic chromatogram and the content of the fructus evodiae standard decoction can be detected at the same time through one-time analysis, the detection time can be effectively shortened, the working efficiency is improved, and a guarantee is provided for quality and consistency evaluation of fructus evodiae formula granules.
Owner:赣江中药创新中心
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com