Method for establishing fingerprint of leech and standard fingerprint of leech
A fingerprint spectrum and a technology for establishing a method, applied in the field of traditional Chinese medicine analysis, can solve the problem that the leech injection or the compound leech injection cannot be accurately and comprehensively reflected, the quality of the medicinal components of the leech cannot be comprehensively and accurately reflected, and the quality of the medicinal components of the leech cannot be accurately and comprehensively reflected. problems such as the quality of leech medicinal materials, to achieve the effect of short test period, less impurities and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The establishment of embodiment 1 leech fingerprint
[0040] 1) Drugs, reagents and instruments
[0041] A total of 10 batches of leech medicinal materials were collected in Baoying, Jiangsu; Agilent1100 high performance liquid chromatography was used; reagents were chromatographically pure or analytically pure;
[0042] 2) Preparation of the test solution
[0043] Accurately weigh 5g of leech coarse powder, put it in a stoppered container, add 50ml of 50% methanol solution precisely, extract in a water bath for 30min, centrifuge at a speed of 10000rpm for 10min, accurately measure 20ml of the supernatant, add to the basic alumina column (6g of alumina, 100-200 mesh, chromatographic column inner diameter of 1.5cm), collect the column liquid, and then elute with 30ml of water, combine the column liquid and water eluate, put it in a 50ml volumetric flask, add water to Scale, shake well, filter through 0.45μm microporous membrane, as the test solution;
[0044] 3) Prepa...
Embodiment 2
[0048] Embodiment 2 leech standard fingerprint
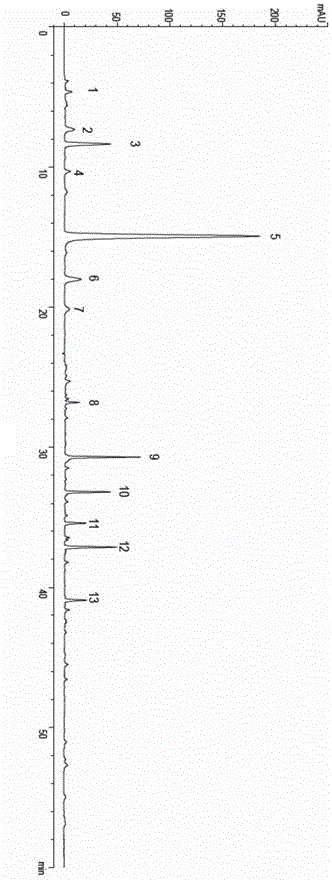
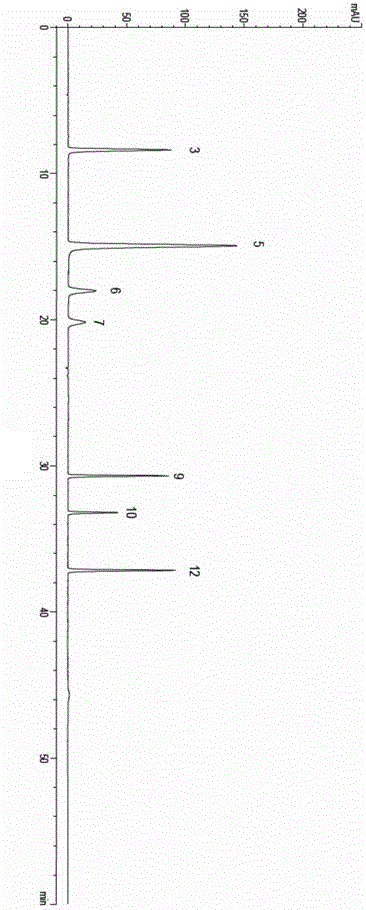
[0049] A total of 10 batches of leech medicinal materials were collected in Baoying, Jiangsu. Obtain each batch of leech fingerprints by the method described in the embodiment of the present invention 1, use the " Chinese medicine chromatographic fingerprint similarity evaluation system 2004 version " software analysis of National Pharmacopoeia Commission, obtain the leech standard fingerprints, determine 13 common characteristic peaks, See figure 1 .
[0050] Chromatographic peaks No. 3, 5, 6, 7, 9, 10, and 12 respectively correspond to uracil, hypoxanthine, xanthine, uridine, hirudamine C, hirudamine B, and hirudamine A respectively, and No. 5 hypoxanthine The purine peak is the strongest, and the total length of the spectrum is 60 minutes, as follows:
[0051] Peak No. 1, the average retention time t is 4.6min;
[0052] Peak No. 2, the average retention time t is 7.3min;
[0053] Peak No. 3, the average retention time t ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mobile phase | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com