Detection method of liquorice formula granules
A technology of formula granule and determination method, applied in measuring devices, instruments, scientific instruments, etc., can solve the problems of extraction of raw materials, inconsistent product technology and quality standards, lack of raw material quality information, and different product quality, etc., and achieve effective product quality. Control, maneuverability, and exclusive effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
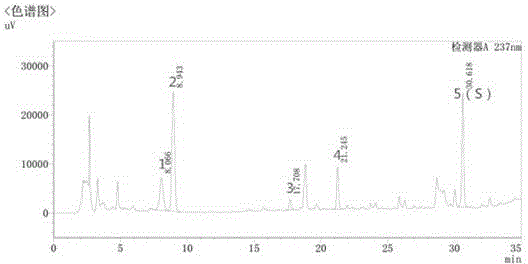
[0031] Embodiment 1: quality control method of licorice formula granule
[0032] (1) Preparation of reference solution
[0033] Take appropriate amount of liquiritin reference substance and ammonium glycyrrhizinate reference substance, weigh them accurately, add 70% ethanol to make solutions containing 20 μg of liquiritin and 0.2 mg of ammonium glycyrrhizinate per 1 ml, respectively, to obtain the reference substance solution.
[0034] (2) Preparation of the test solution
[0035] Take 0.1g of licorice formula granule powder, weigh it accurately, put it in a stoppered Erlenmeyer flask, add 100ml of 70% ethanol precisely, seal it tightly, weigh it, treat it with ultrasound for 30 minutes, let it cool, weigh it again, and use 70% Make up the lost weight with ethanol, shake well, filter, and take the filtrate to obtain the test solution.
[0036] The chromatographic conditions determined by high performance liquid chromatography are:
[0037] The chromatographic column is Wate...
Embodiment 2
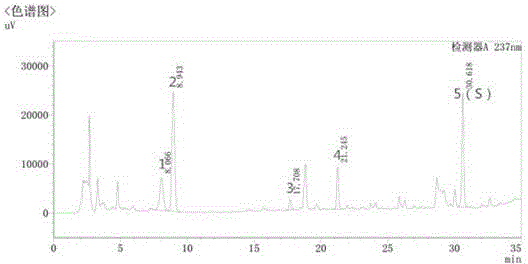
[0044] Embodiment 2: quality control method of licorice formula granule
[0045] (1) Preparation of reference solution
[0046] Take appropriate amount of liquiritin reference substance and ammonium glycyrrhizinate reference substance, weigh them accurately, add 70% ethanol to make solutions containing 20 μg of liquiritin and 0.2 mg of ammonium glycyrrhizinate per 1 ml, respectively, to obtain the reference substance solution.
[0047] (2) Preparation of the test solution
[0048] Take 0.05g of licorice formula granule powder, weigh it accurately, put it in a stoppered Erlenmeyer flask, add 50ml of 70% ethanol precisely, seal it tightly, weigh it, treat it ultrasonically for 10 minutes, let it cool, weigh it again, and use 70% Make up the lost weight with ethanol, shake well, filter, and get the continued filtrate to obtain the test solution;
[0049] The chromatographic conditions determined by high performance liquid chromatography are:
[0050] The chromatographic column...
Embodiment 3
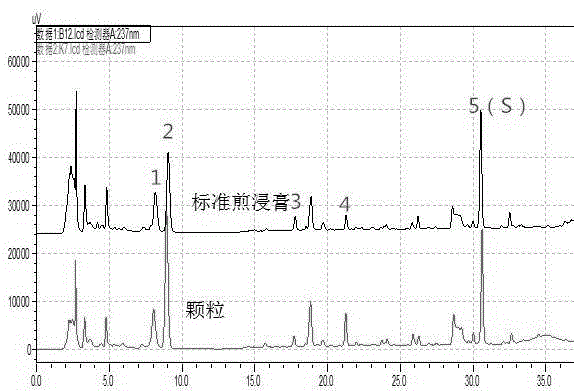
[0057] Embodiment 3: quality control method of licorice formula granule
[0058] (1) Preparation of reference solution
[0059]Take appropriate amount of liquiritin reference substance and ammonium glycyrrhizinate reference substance, weigh them accurately, add 70% ethanol to make solutions containing 20 μg of liquiritin and 0.2 mg of ammonium glycyrrhizinate per 1 ml, respectively, to obtain the reference substance solution.
[0060] (2) Preparation of the test solution
[0061] Take 0.2g of licorice formula granule powder, weigh it accurately, put it in a stoppered Erlenmeyer flask, add 100ml of 70% ethanol accurately, seal it tightly, weigh it, treat it ultrasonically for 40 minutes, let it cool, weigh it again, and use 70% Make up the lost weight with ethanol, shake well, filter, and take the filtrate to obtain the test solution.
[0062] The chromatographic conditions determined by high performance liquid chromatography are:
[0063] The chromatographic column is Water...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com