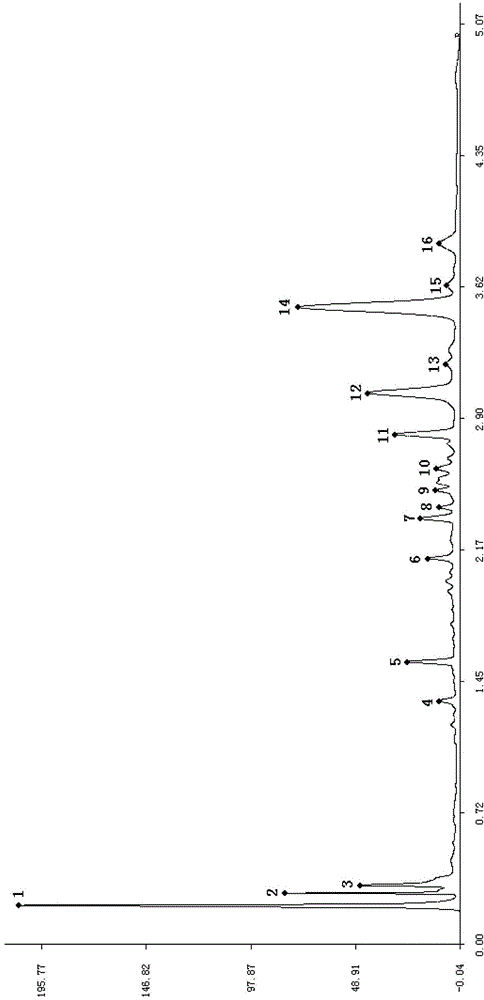
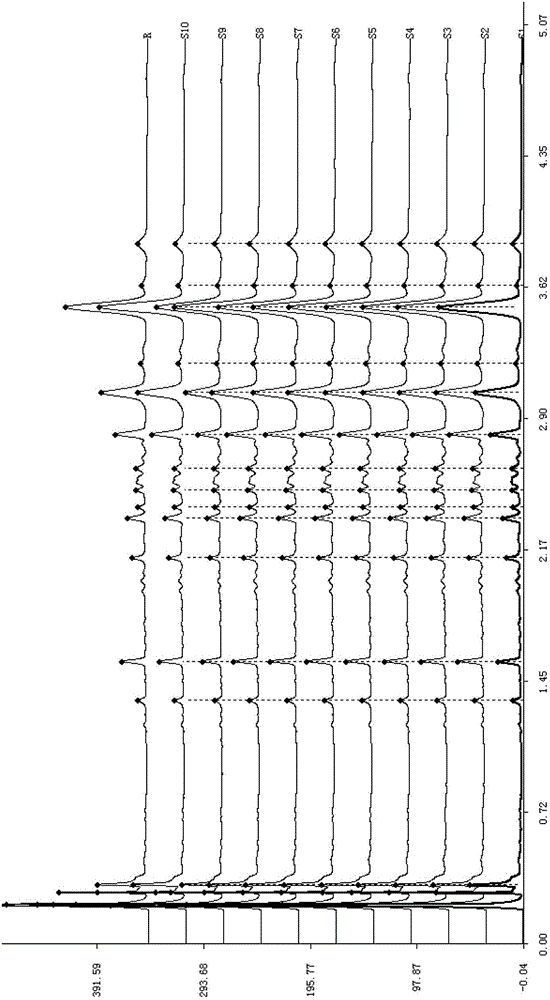
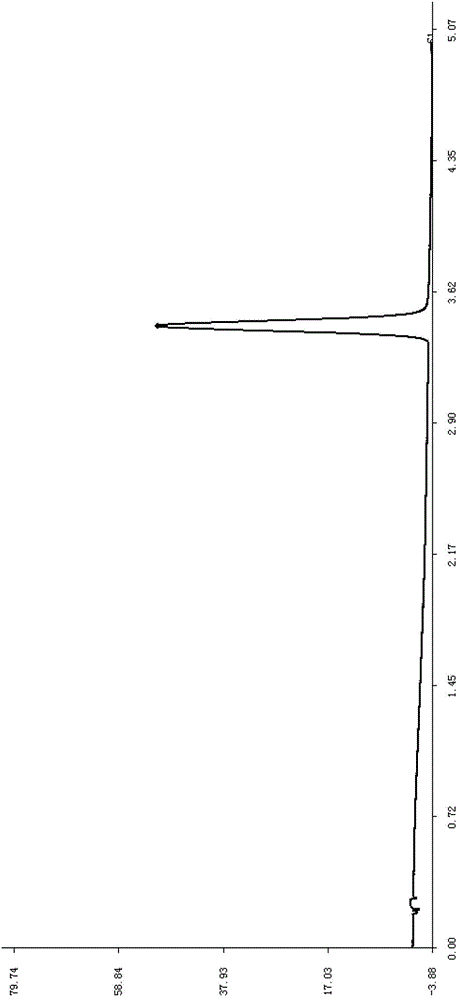
Construction method of UPLC fingerprint of sweet clover medicinal material and standard fingerprint thereof
A standard fingerprint and construction method technology, applied in the field of drug analysis, can solve the problems of not being able to effectively reflect and control the overall quality of Medicinal Rhinoceros, and achieve the effects of shortening online analysis time, high precision, and more peaks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1. Instruments and medicines
[0041] Use WatersACQUITYUPLCH-Class ultra-high performance liquid chromatography. A total of 10 batches of sweet rhinoceros medicinal materials were purchased from Anhui Mengshi Pharmaceutical Co., Ltd., and the coumarin reference substance (EP standard) was purchased from ChromaDax Co., Ltd. (ChromaDax, Inc.). Other reagents were chromatographically pure or analytically pure.
[0042] 2. Preparation of test sample solution
[0043] Precisely weigh 2.0000g of medicinal material powder, put it in a stoppered container, add 90mL of 70% by volume methanol aqueous solution to soak, oscillate ultrasonically for 30 minutes, let cool, filter, collect medicinal residues, repeat the extraction once and combine twice according to the above method The extracted filtrate was put into a 250ml measuring flask, adjusted to the mark with methanol-water (volume ratio 1:1), and filtered with a 0.22 μm filter membrane, and the filtrate was used as the test ...
Embodiment 2
[0058] 1. Instruments and medicines used
[0059] Use WatersACQUITYUPLCH-Class ultra-high performance liquid chromatography. A total of 10 batches of Medicinal Rhizoma Medicinae were purchased from Anhui Mengshi Pharmaceutical Co., Ltd., the coumarin reference substance (EP standard) was purchased from ChromaDax Co., Ltd. (ChromaDax, Inc.), and other reagents were of chromatographic or analytical grade reagent.
[0060] 2. Preparation of test sample solution
[0061] Accurately weigh 2.0000g of sweet rhinoceros medicinal powder, put it in a stoppered container, add 90mL of 50% methanol aqueous solution to soak, oscillate ultrasonically for 30 minutes, let cool, filter, then collect the medicinal residues, repeat the extraction once again according to the above method, and combine Put the filtrate extracted twice into a 250mL volumetric flask, dilute to the mark with methanol-water (volume ratio 1:1), filter with a 0.22μm filter membrane, and use the filtrate as the test samp...
Embodiment 3
[0088] 1. Instruments and medicines
[0089] Use WatersACQUITYUPLCH-Class ultra-high performance liquid chromatography. A total of 10 batches of sweet rhinoceros medicinal materials were purchased from Anhui Mengshi Pharmaceutical Co., Ltd., and the coumarin reference substance (EP standard) was purchased from ChromaDax Co., Ltd. (ChromaDax, Inc.). Other reagents were chromatographically pure or analytically pure.
[0090] 2. Preparation of test sample solution
[0091] Accurately weigh 2.0000g of medicinal material powder, put it in a stoppered container, add 90mL of 30% by volume methanol aqueous solution to soak, oscillate ultrasonically for 30 minutes, let cool, filter, collect medicinal residues, repeat the extraction once and combine twice according to the above method The extracted filtrate was put into a 250ml measuring flask, adjusted to the mark with methanol-water (volume ratio 1:1), and filtered with a 0.22 μm filter membrane, and the filtrate was used as the test...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com