Method for establishing quantitative fingerprint spectrum of standard fructus evodiae decoction
A fingerprint and Evodia technology, which is applied in the field of establishing the quantitative fingerprint of Evodia standard decoction, can solve the problems of providing reference for the quality control of difficult Evodia formula granules, unable to meet the quality control requirements of Evodia, analysis time and efficiency constraints, etc., and achieve stability. and good reproducibility, easy realization of chromatographic conditions, and reduction of detection costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
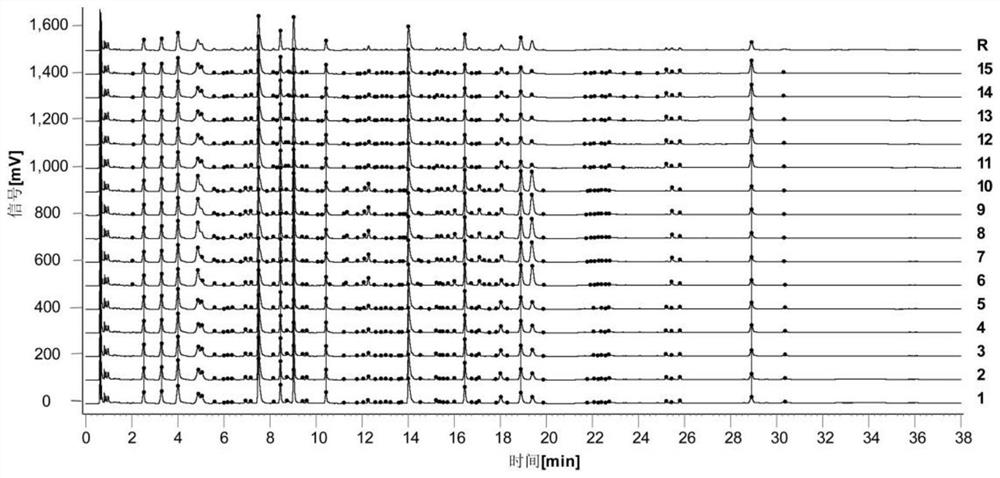
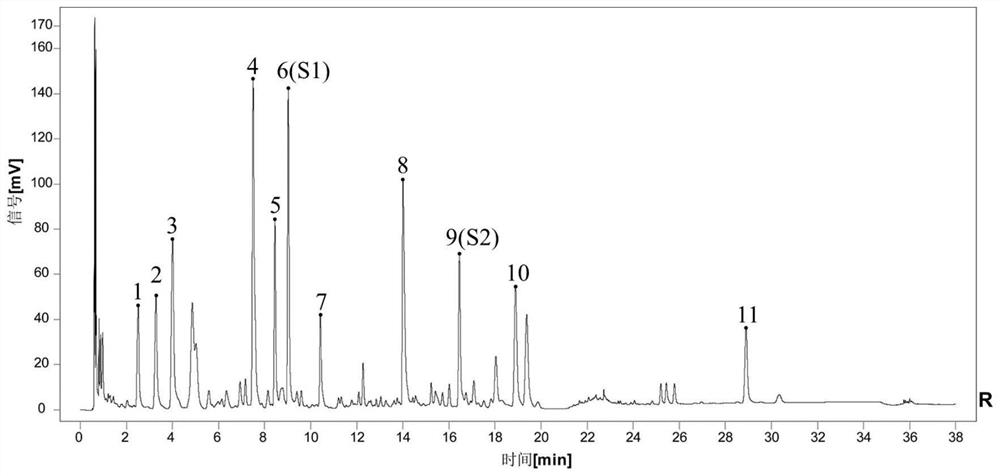
[0042] Example 1: Establishment of UPLC characteristic map method for Evodia (Shihu) standard decoction
[0043] 1.1 Instruments, reagents, reagents and sources
[0044] Instruments: Waters Acquity I Class ultra-performance liquid chromatograph; Agilent Infinity II1290-6545Q-TOF LC-MS Agilent ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometer; ML204T / 02 1 / 00000 analytical balance; XSE105 ten 1 / 10,000 analytical balance; KQ-500DV ultrasonic instrument; Waters Acquity UPLC BEH ShieldRP18 (2.1×100mm, 1.7μm) chromatographic column and pre-column; SOrvall ST8 high-speed centrifuge;
[0045] Reagents: acetonitrile (Thermo) and formic acid (Thermo) are mass spectrometry grade; phosphoric acid (Aladdin) and acetonitrile (Fisher) are chromatography grade; methanol (General-Reagent) is analytical grade; ultrapure water (Milli-QIQ 7000);
[0046] Reagents: chlorogenic acid (batch number: 110753-202018, specification: 20mg, purity: 96.1%), hypericin (batc...
example 2
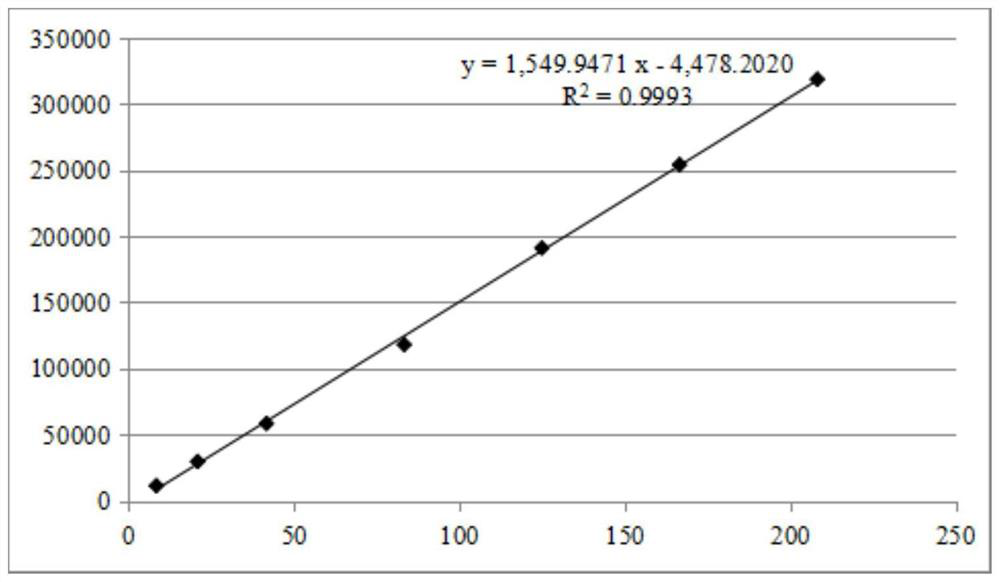
[0083] Example 2: Establishment of the content determination method of Evodia (Shihu) standard decoction
[0084] 1.1 Instruments, reagents, reagents and sources
[0085] Instrument: Waters Acquity I Class ultra-high performance liquid chromatograph; ML204T / 02 1 / 10,000 analytical balance; XSE105 1 / 100,000 analytical balance; KQ~500DV ultrasonic instrument; Waters Acquity UPLC BEH ShieldRP18 (2.1×100mm, 1.7μm ) chromatographic column and pre-column; SOrvall ST8 high-speed centrifuge;
[0086] Reagents: formic acid (Macklin) and acetonitrile (Fisher) are chromatographic grade; methanol (Energy Chemical) is analytical grade; ultrapure water;
[0087] Reagents: limonin (batch number 110800-201707, specification 20mg, purity 97.9%) was purchased from China National Institute for Food and Drug Control; Hospital; Evodia (batch number 110801-2021109, specification 20mg, purity 99. Macklin 3%) was purchased from China National Institute for Food and Drug Control.
[0088] The source...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com