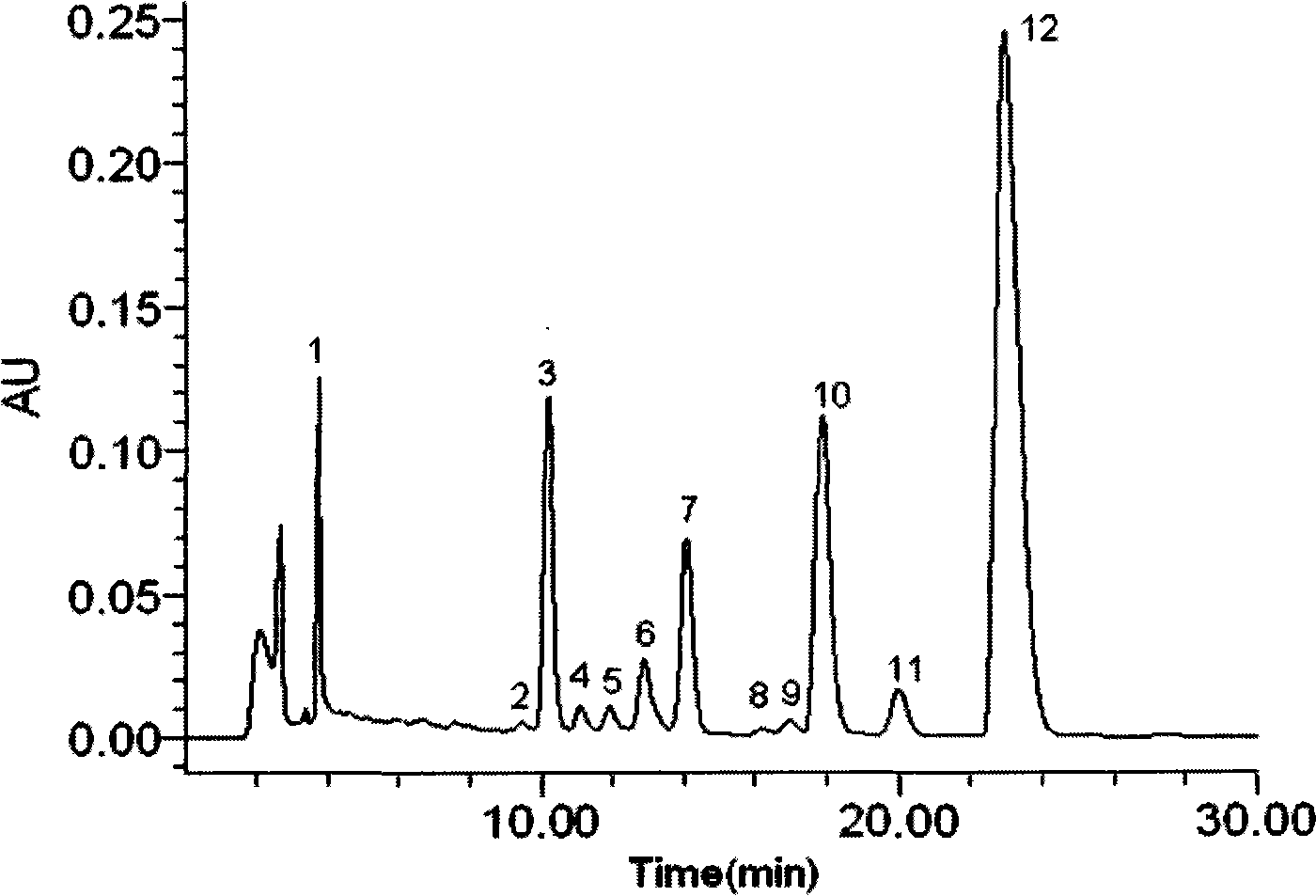
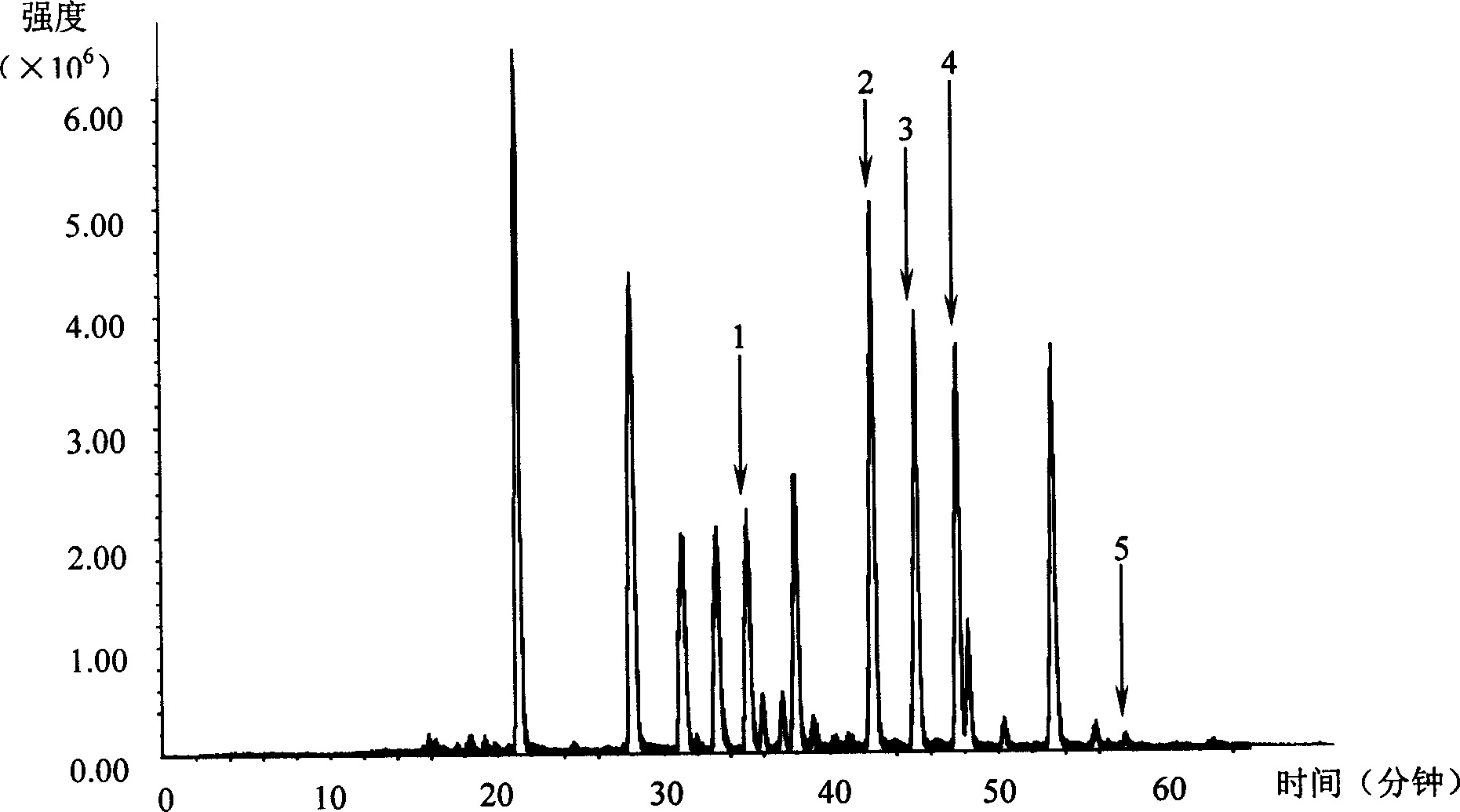
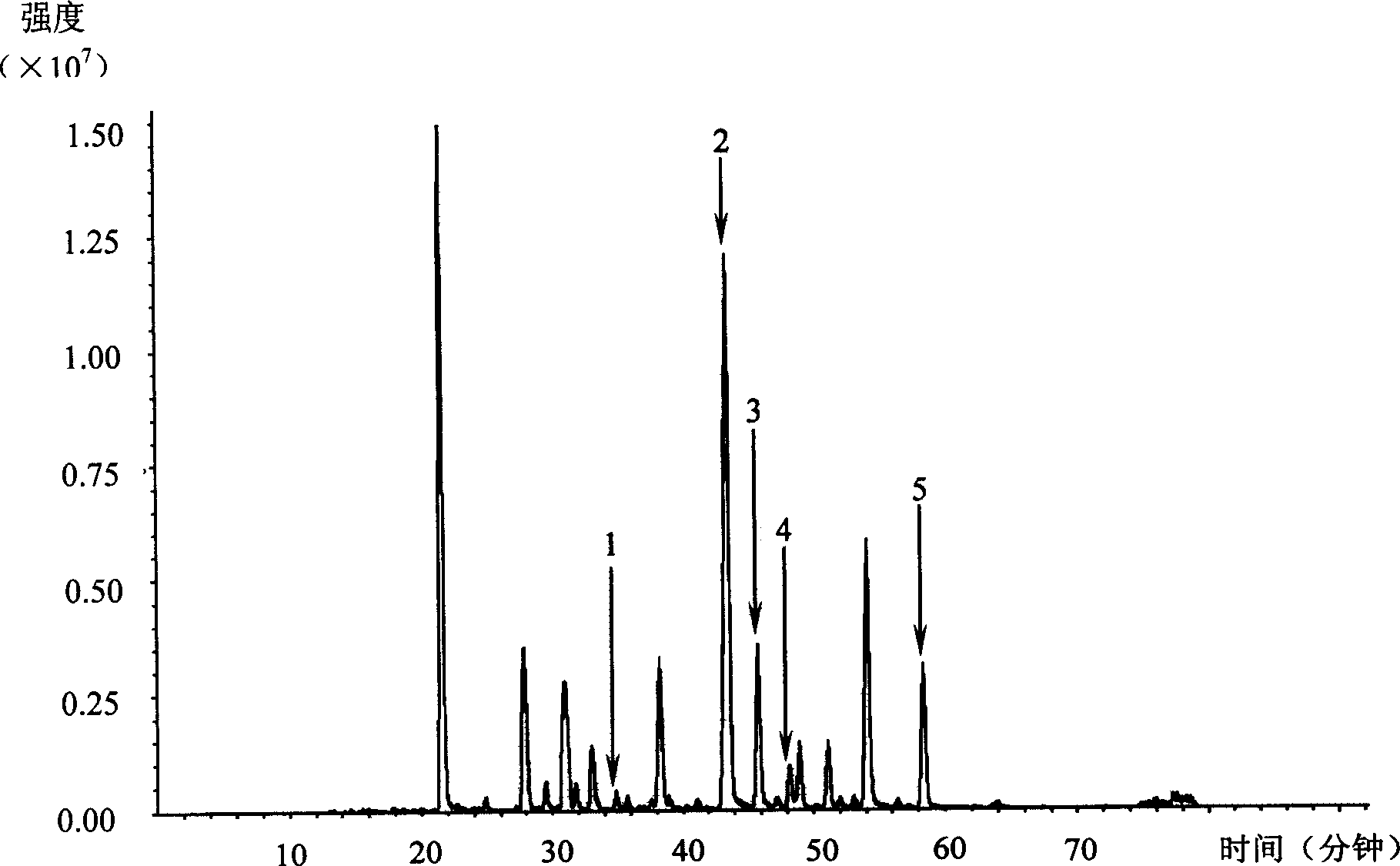
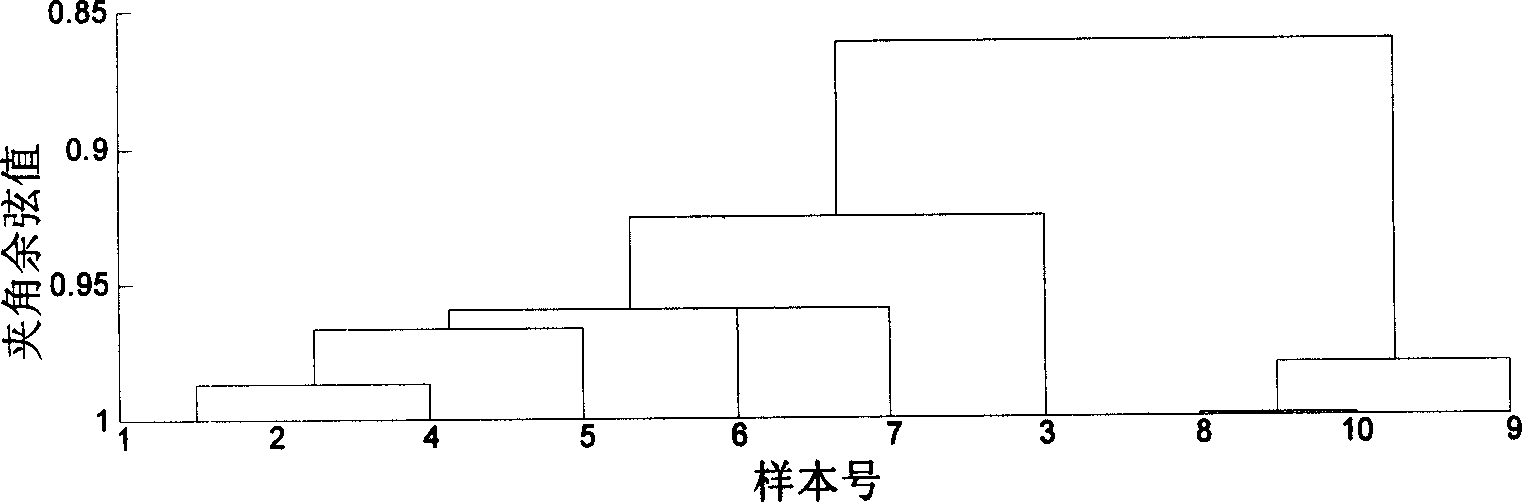
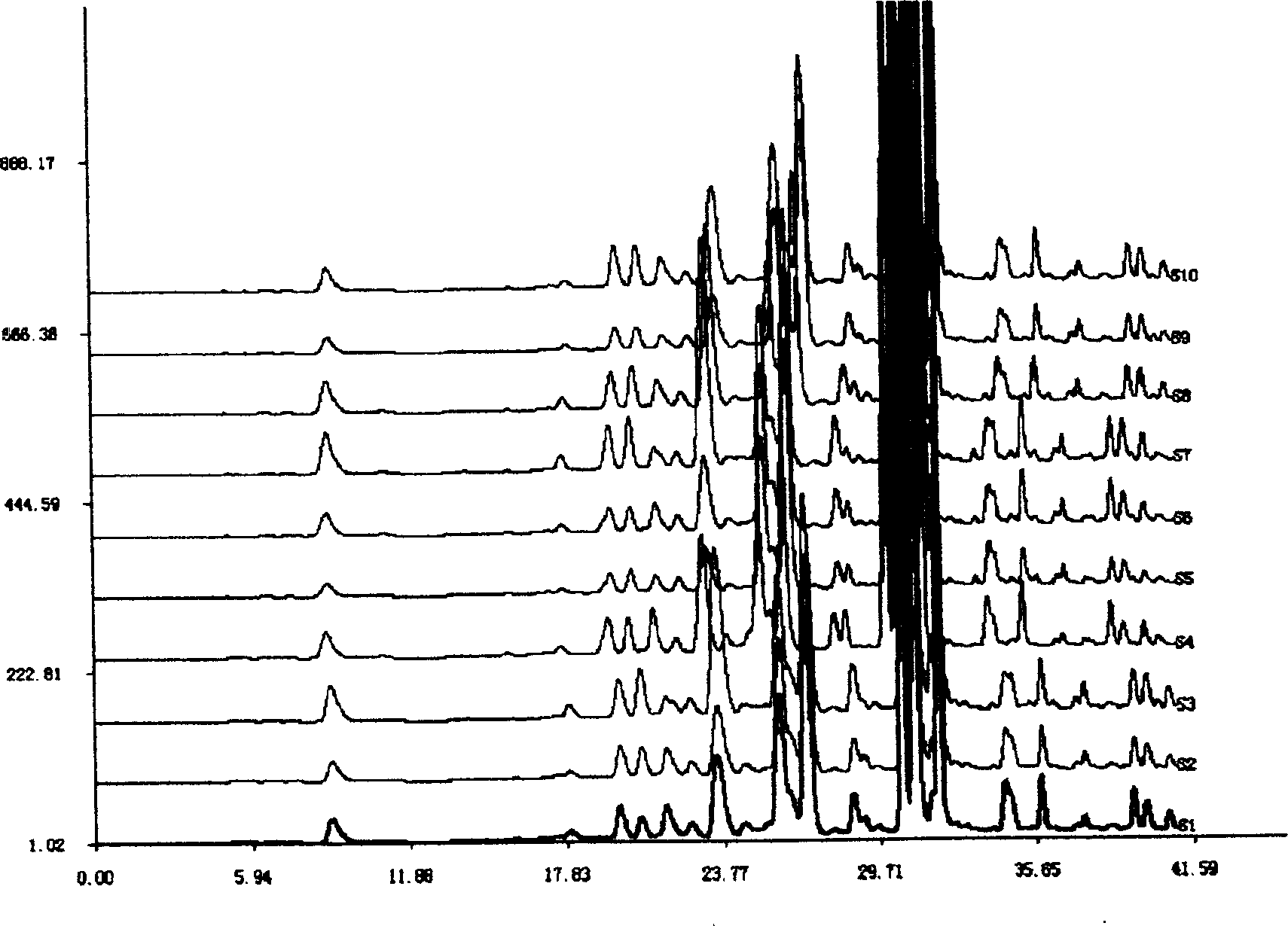
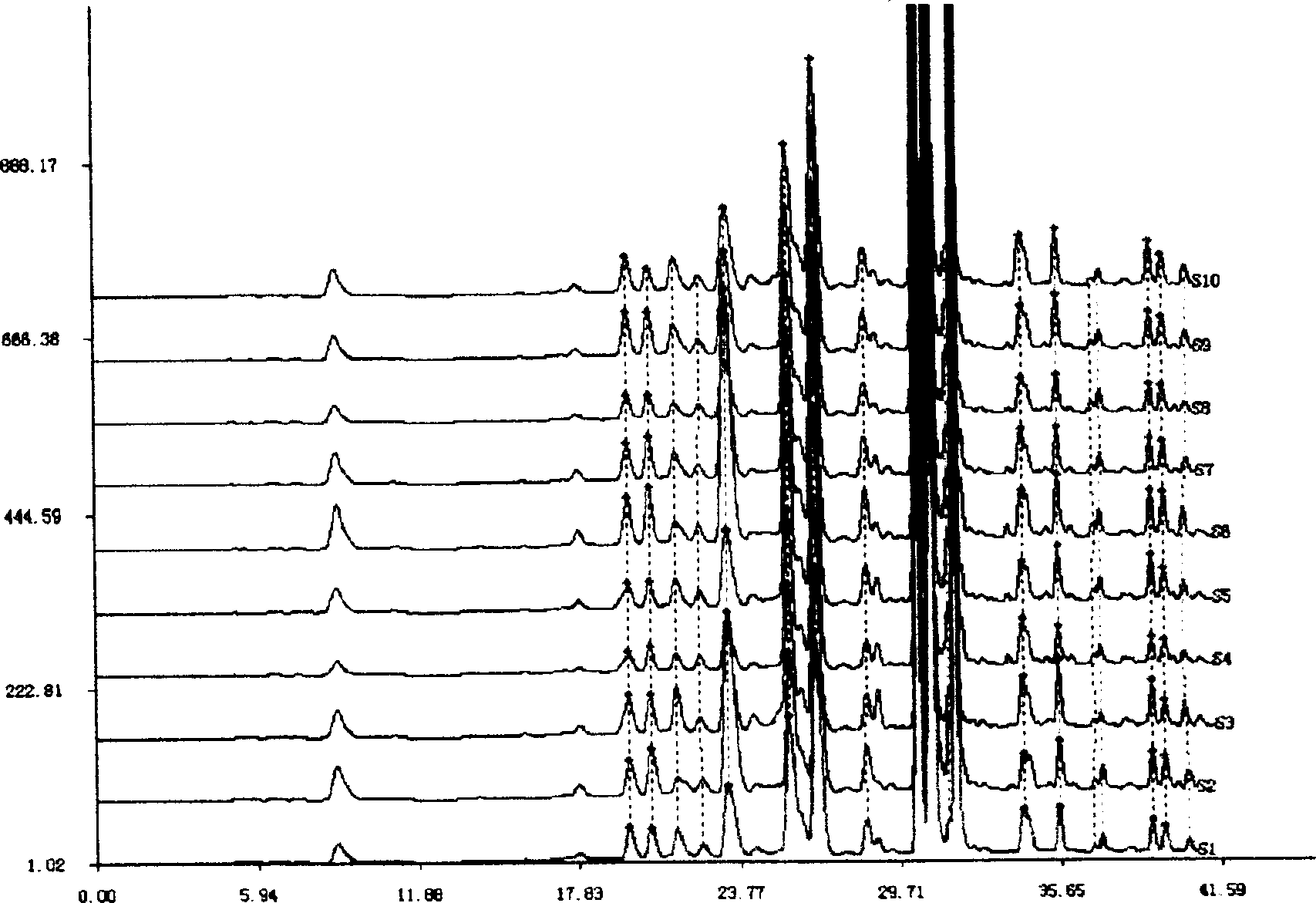
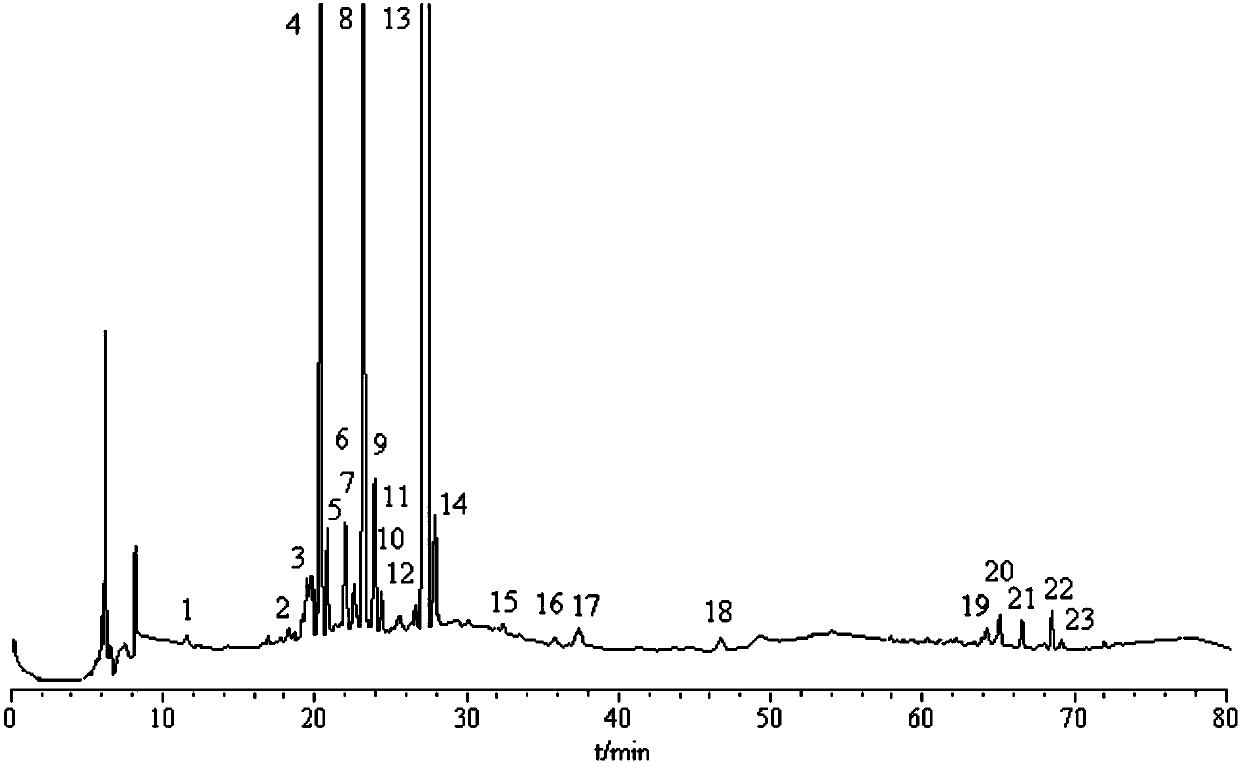
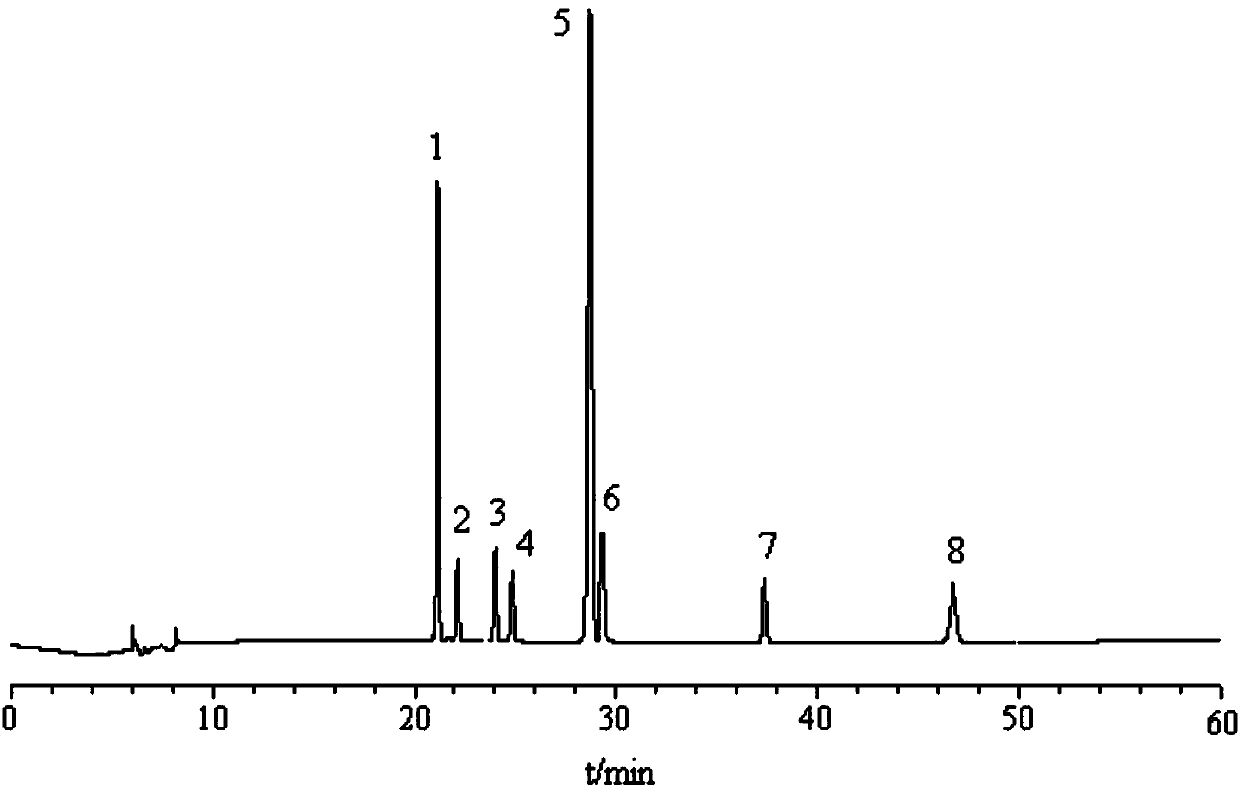
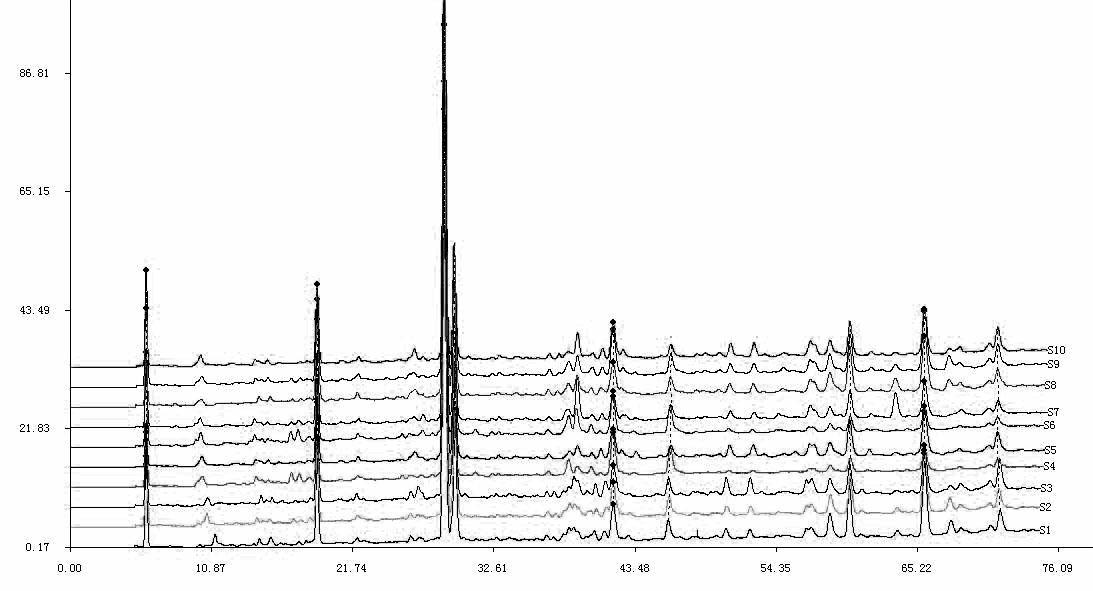
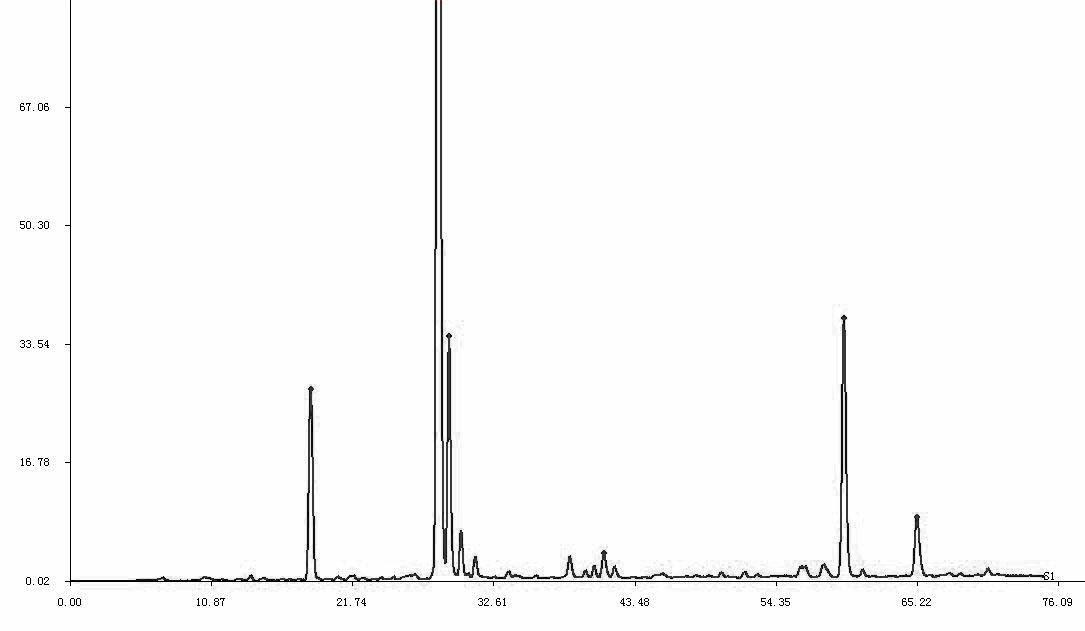
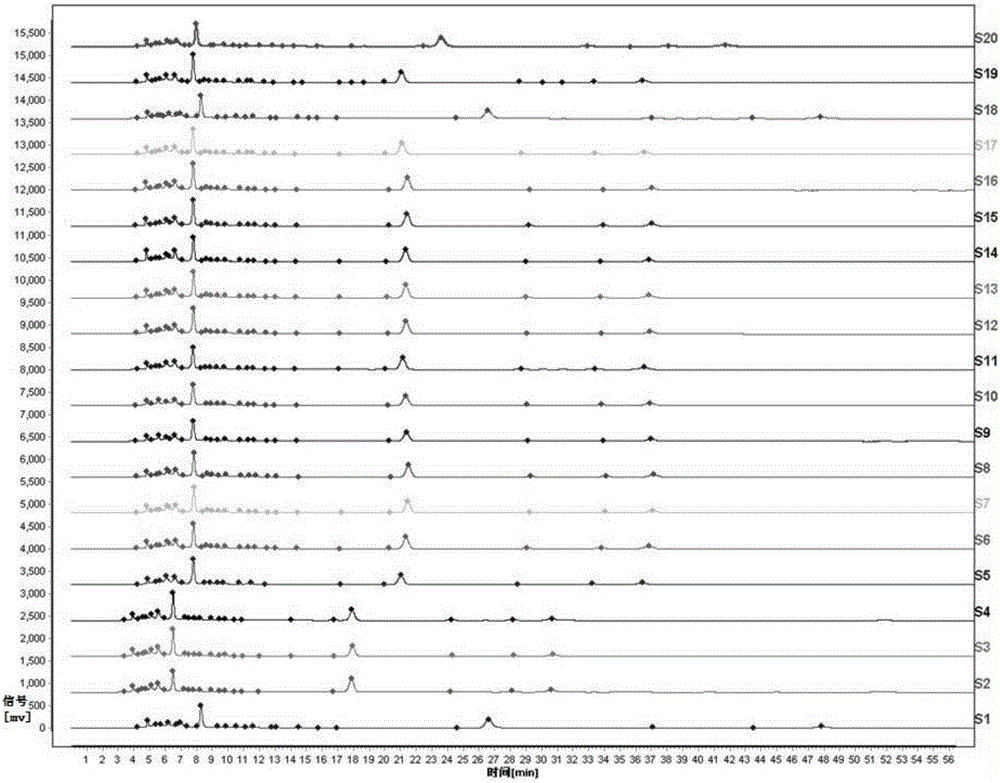
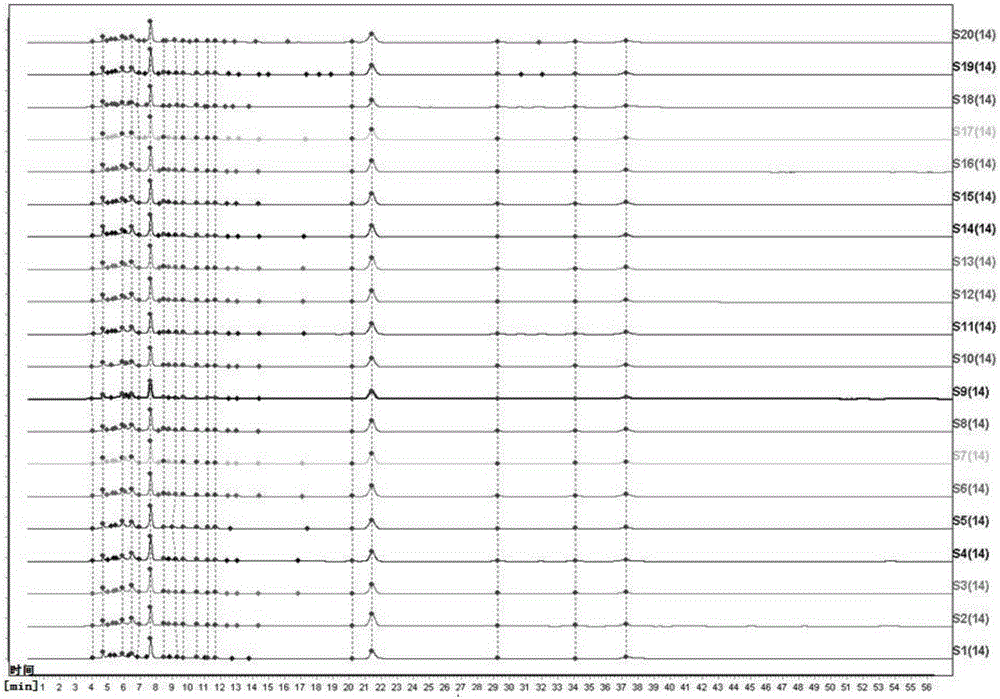
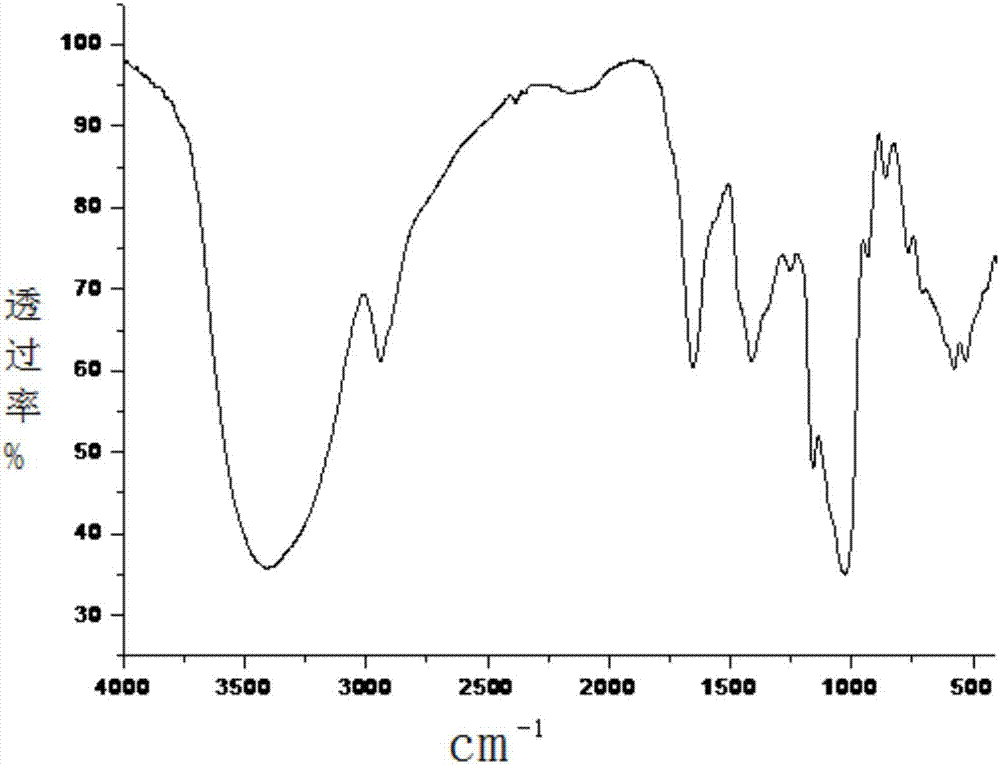
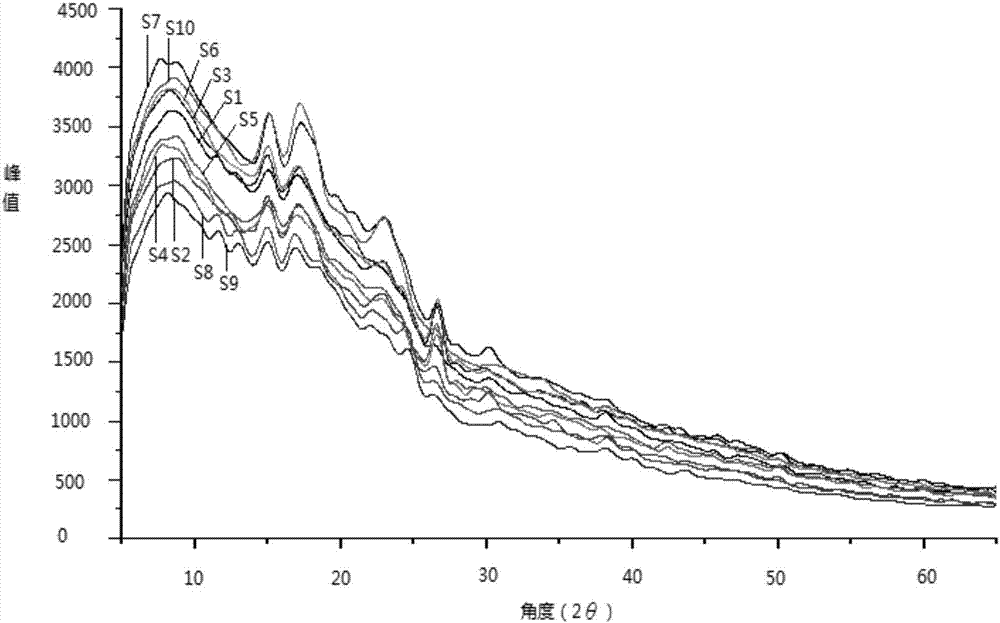
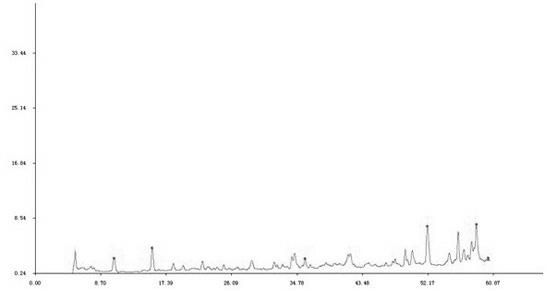
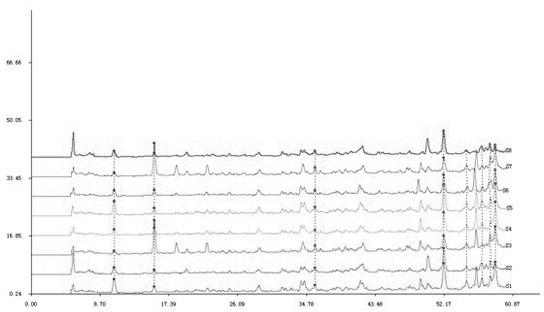
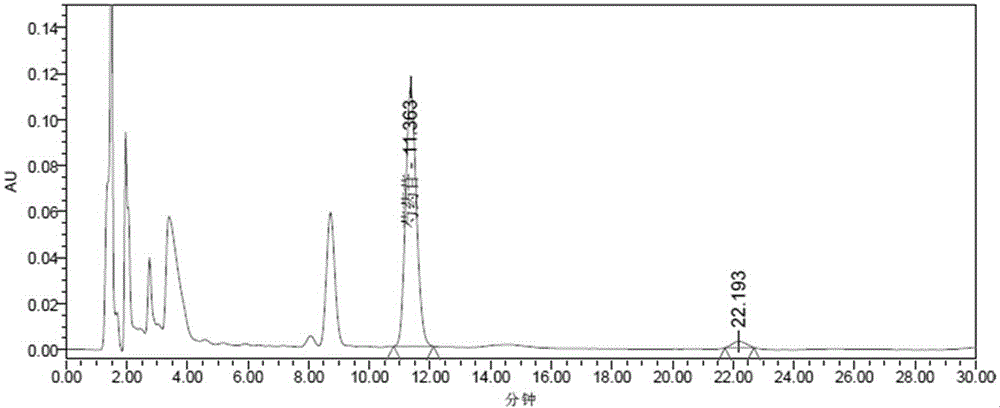
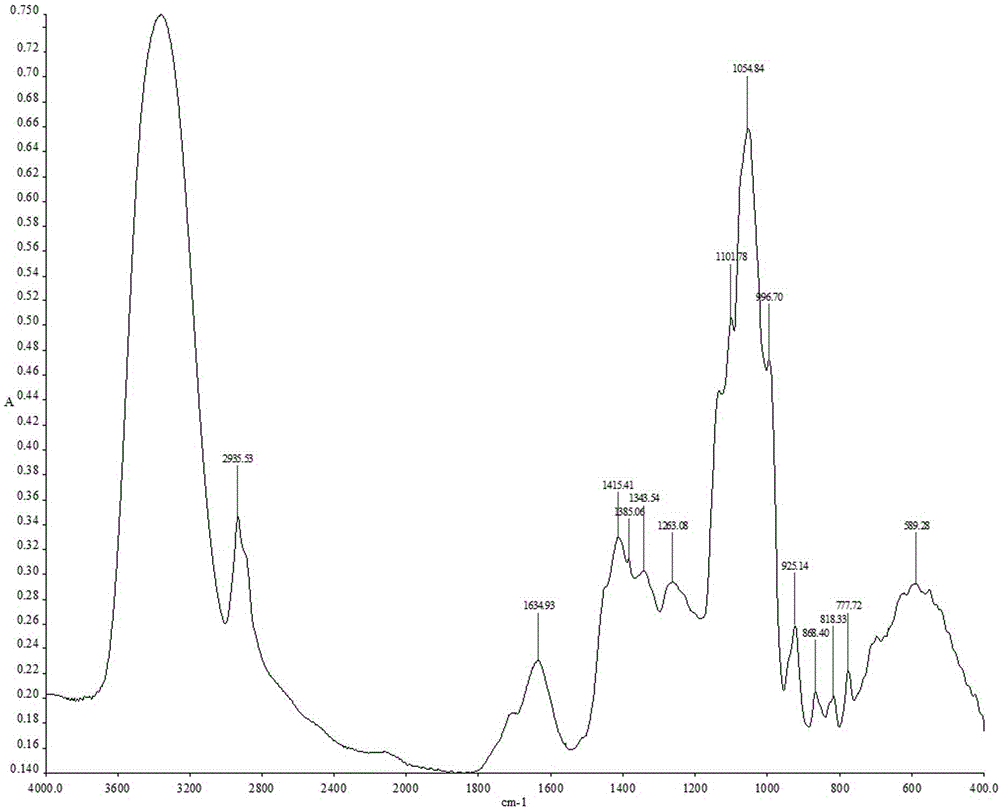
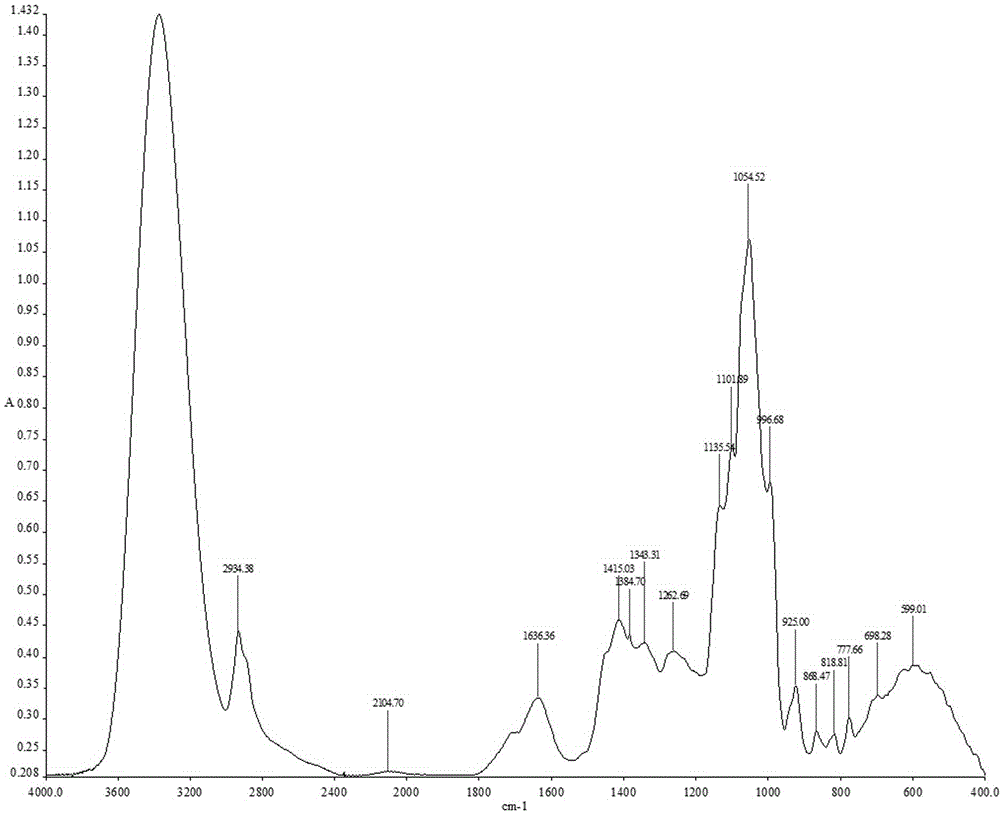
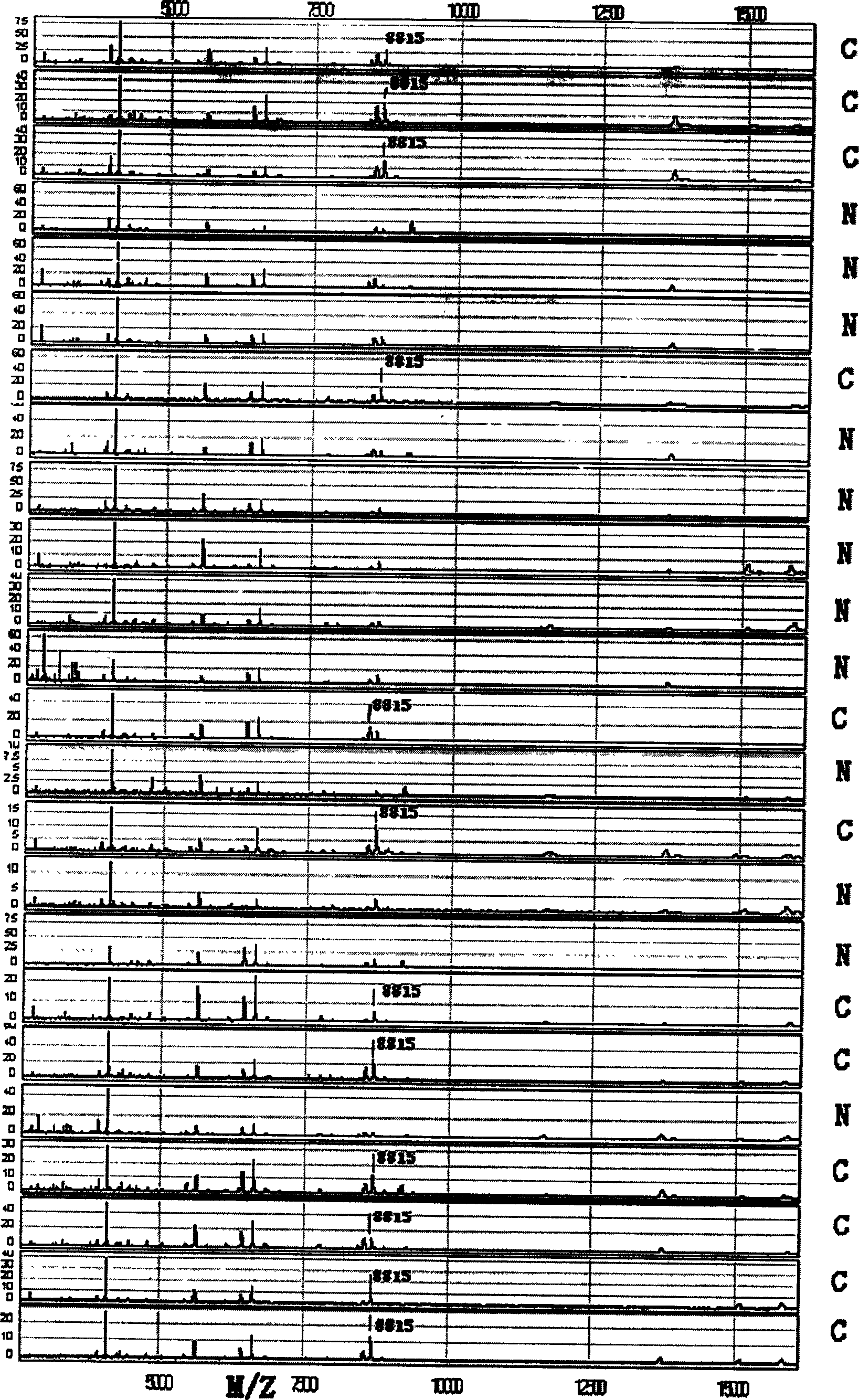
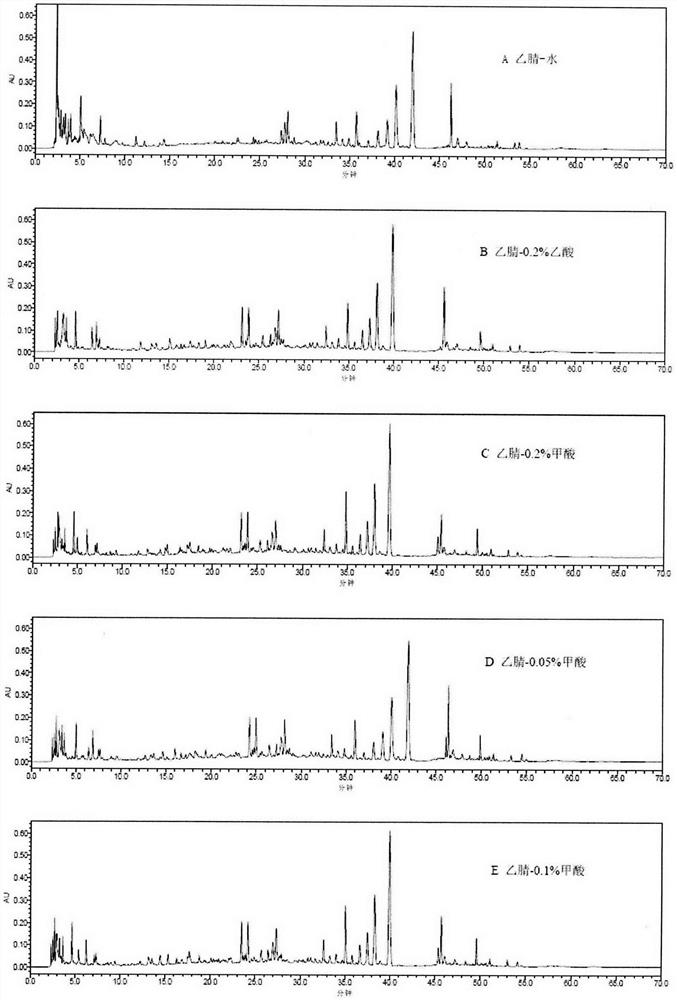
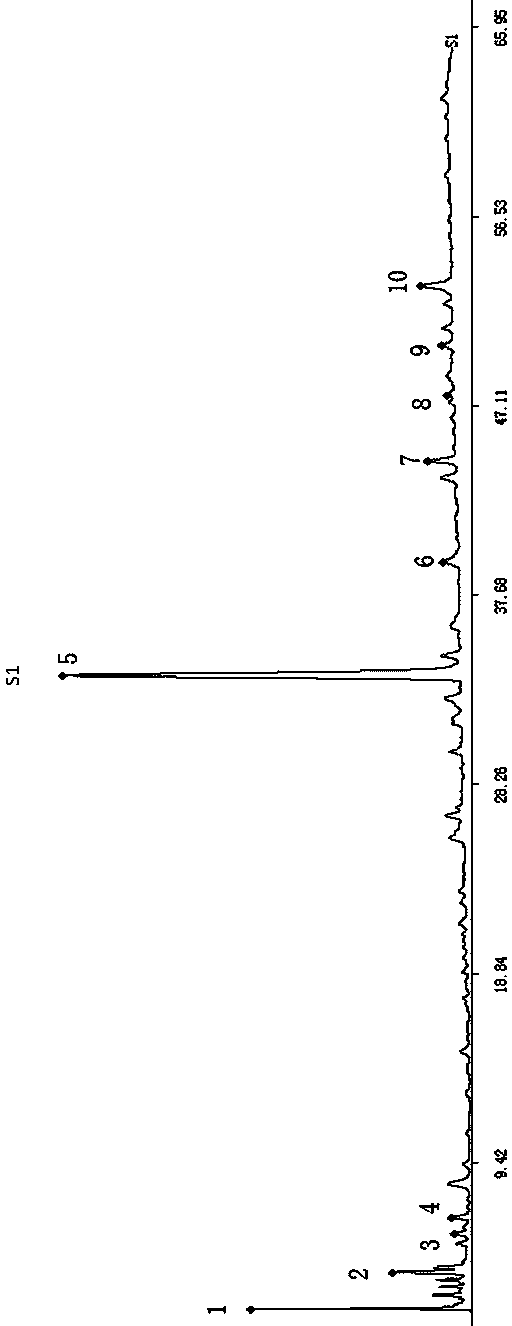
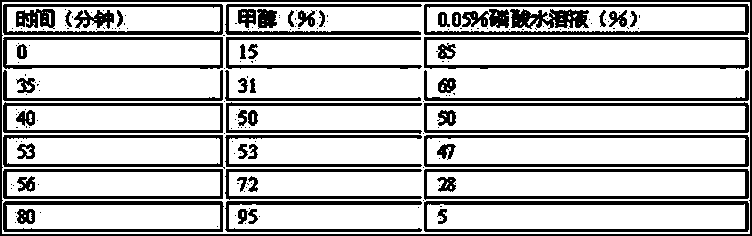
Patents
Literature
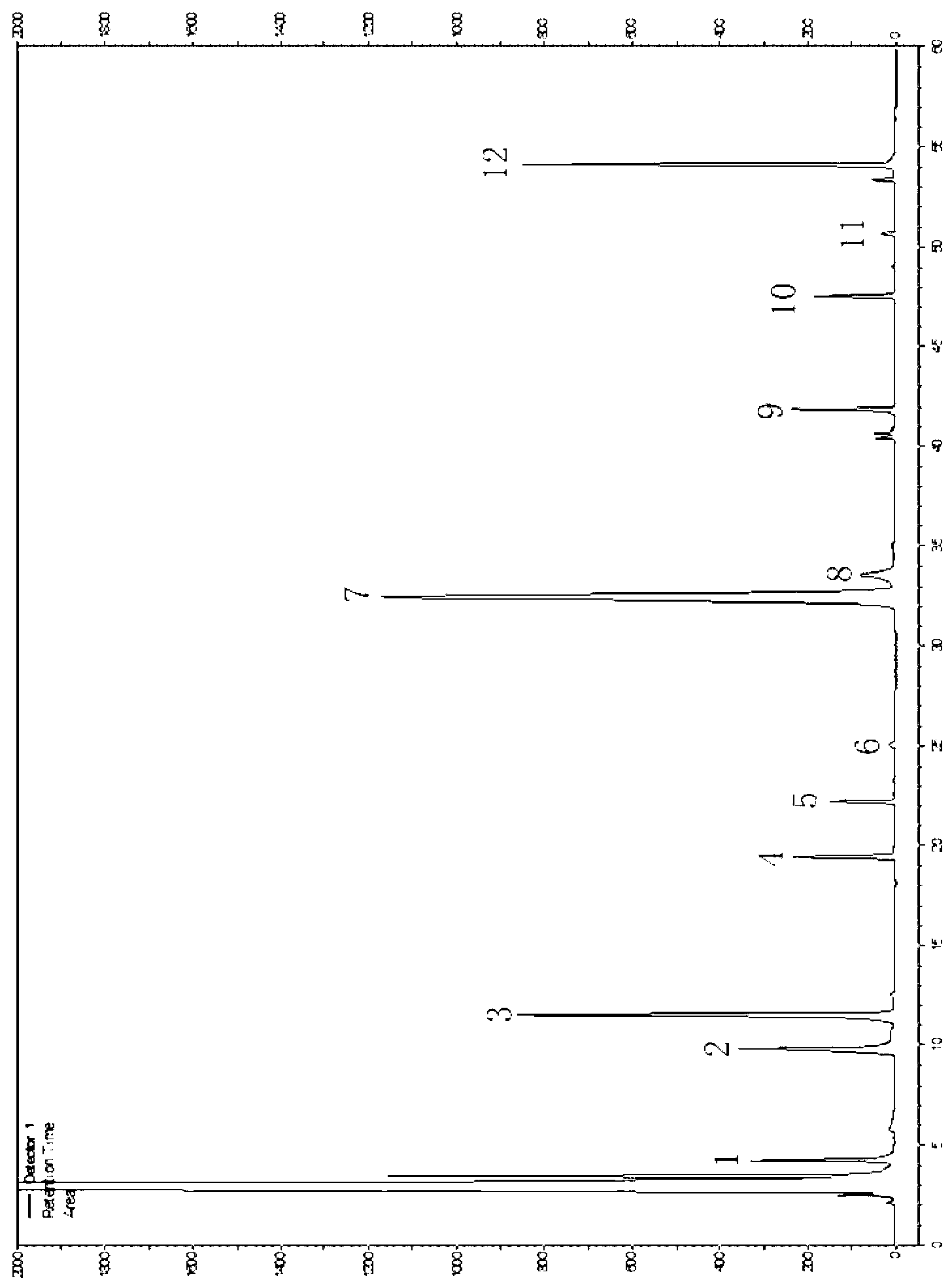
217 results about "Chemogenetics" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chemogenetics is the processes by which macromolecules can be engineered to interact with previously unrecognized small molecules. Chemogenetics as a term was originally coined to describe the observed effects of mutations on chalcone isomerase activity on substrate specificities in the flowers of Dianthus caryophyllus. This method is very similar to optogenetics however, it uses chemically engineered molecules and ligands instead of light and light-sensitive channels known as Opsins.
Method for building helicobacter pylori nucleic acid fingerprint spectrum and product thereof
ActiveCN102827930AAccurate identificationMicrobiological testing/measurementMicroorganism based processesEnzymatic digestionMass spectrometry
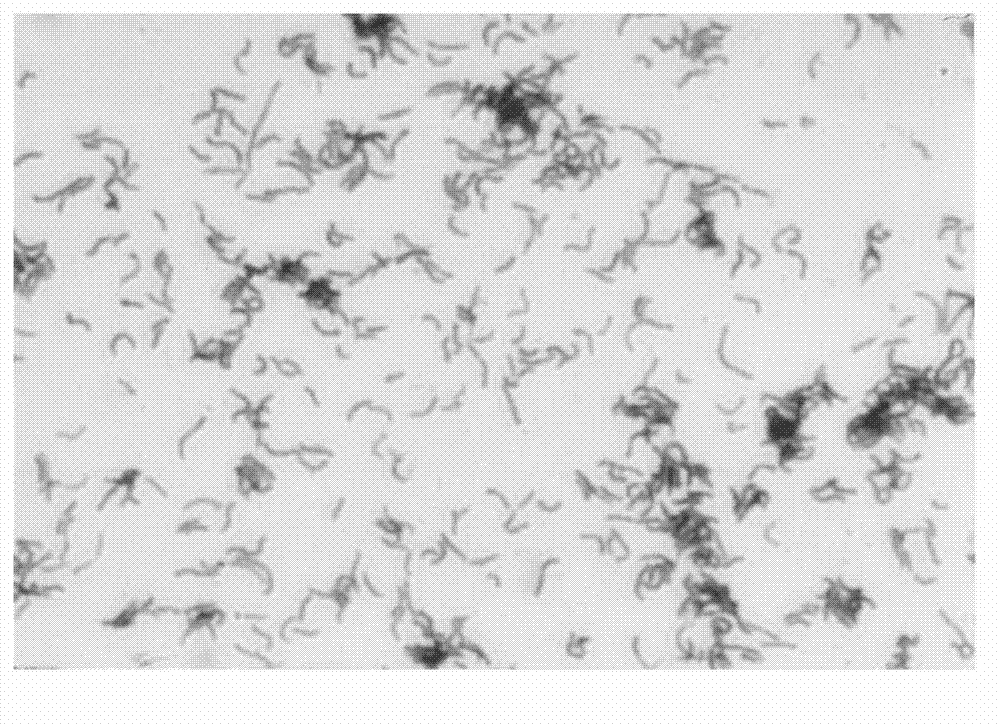
The invention discloses a method for building a helicobacter pylori nucleic acid fingerprint spectrum, which comprises PCR (polymerase chain reaction) amplification, SAP (severe acute pancreatitis) enzymatic digestion, transcription and nuclease digestion, purification, mass spectrometer detection and the like. A helicobacter pylori nucleic acid fingerprint spectrum database is set up on the basis of the method. According to the generated mass peak spectrum of the experiment, the helicobacter pylori of a sample to be detected can be quickly identified, so the method can be widely applied in the fields of helicobacter pylori types and classification, environmental sanitation, public safety qunarantine and the like.
Owner:BIOYONG TECH
Fingerprint pattern quality control method for cordyceps sinensis bacterium powder raw material in herbs medicaments for strengthening the body resistance and activating blood and dissolving stasis
ActiveCN101293002AGuarantee normal implementationHigh sensitivityFungiComponent separationHplc fingerprintRetention time
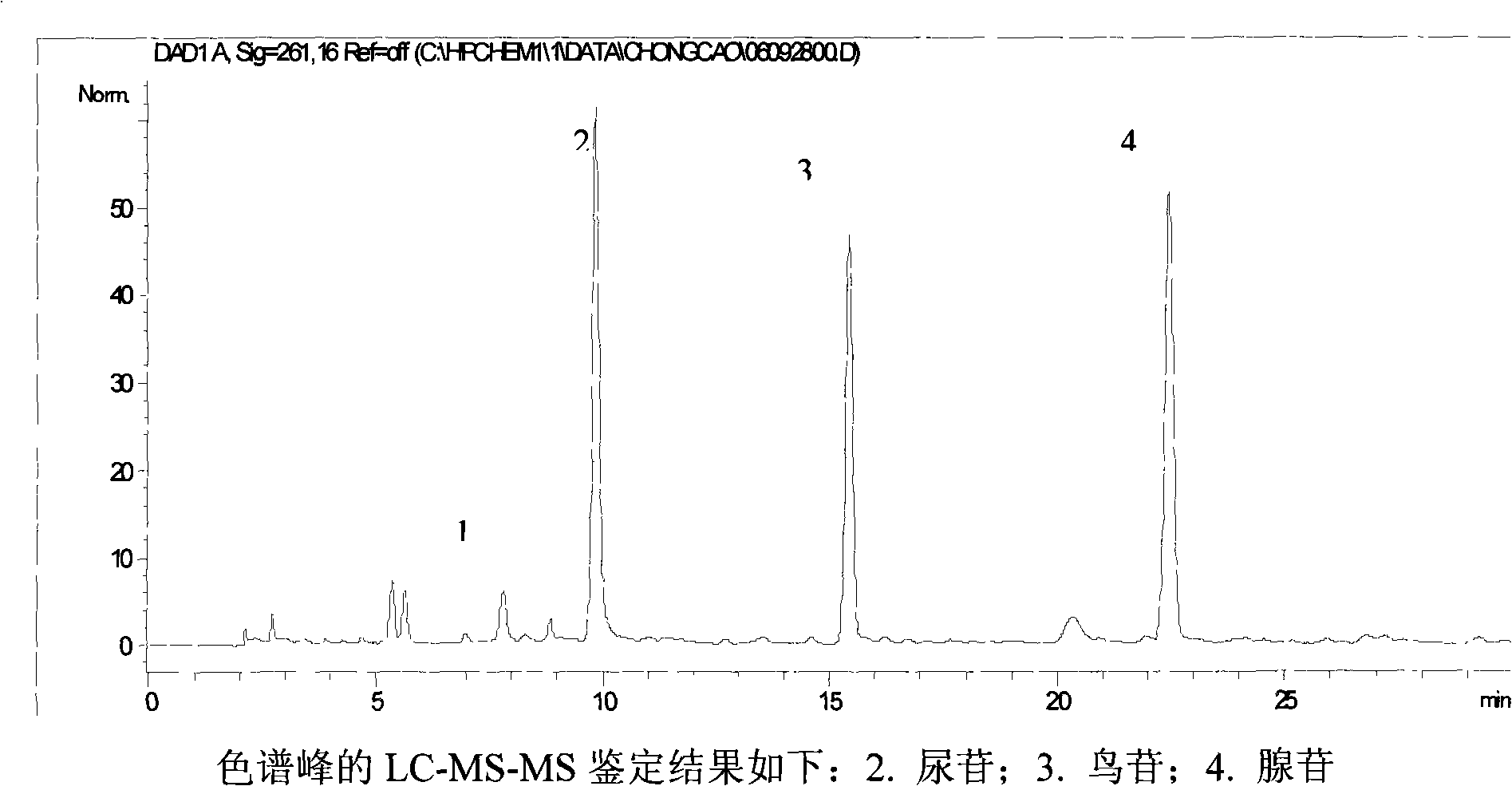
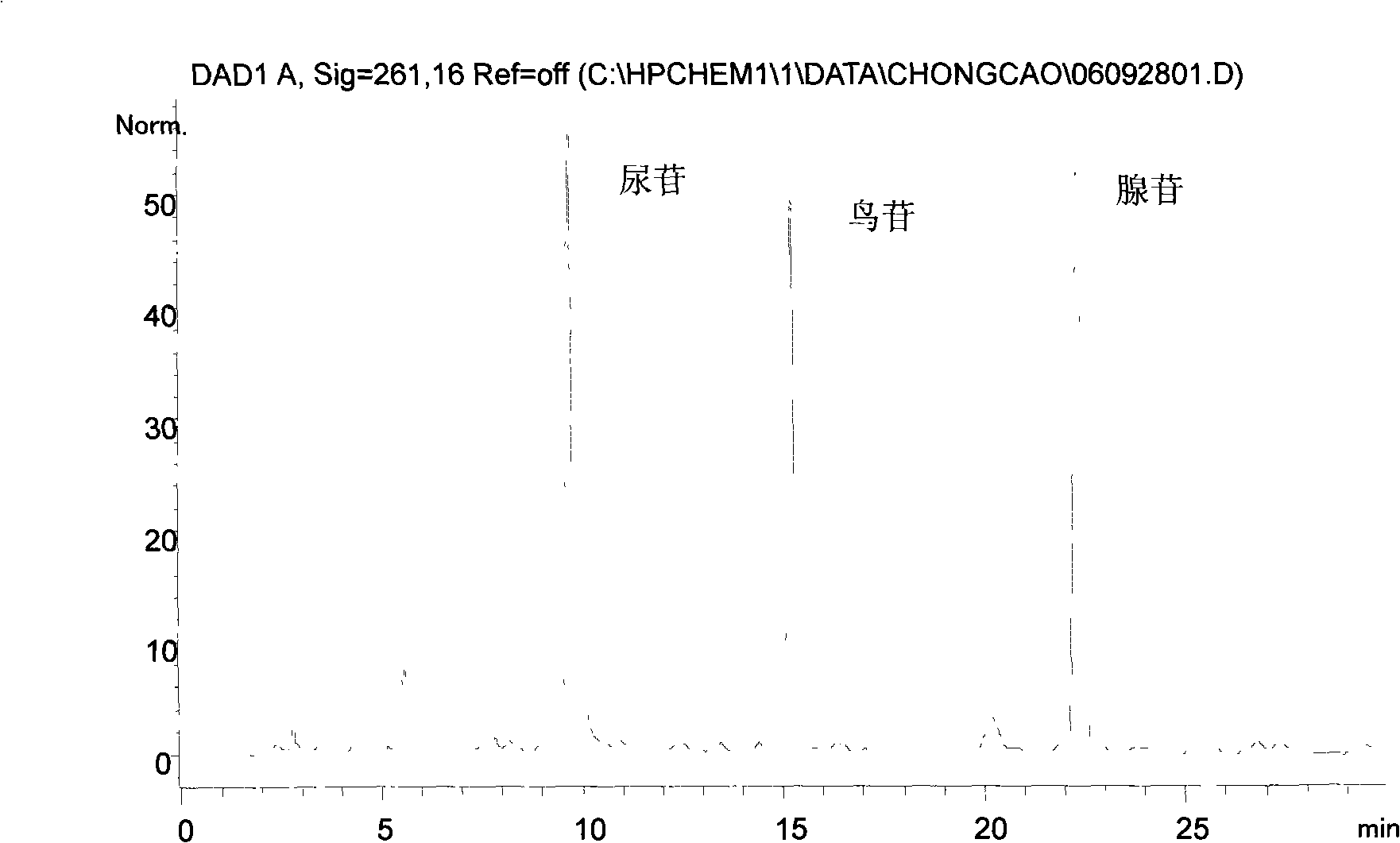
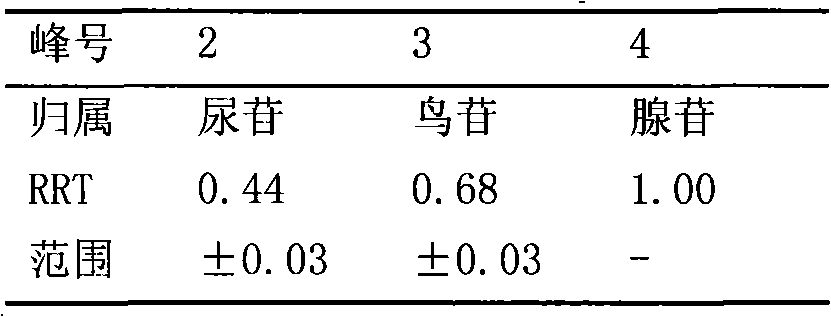
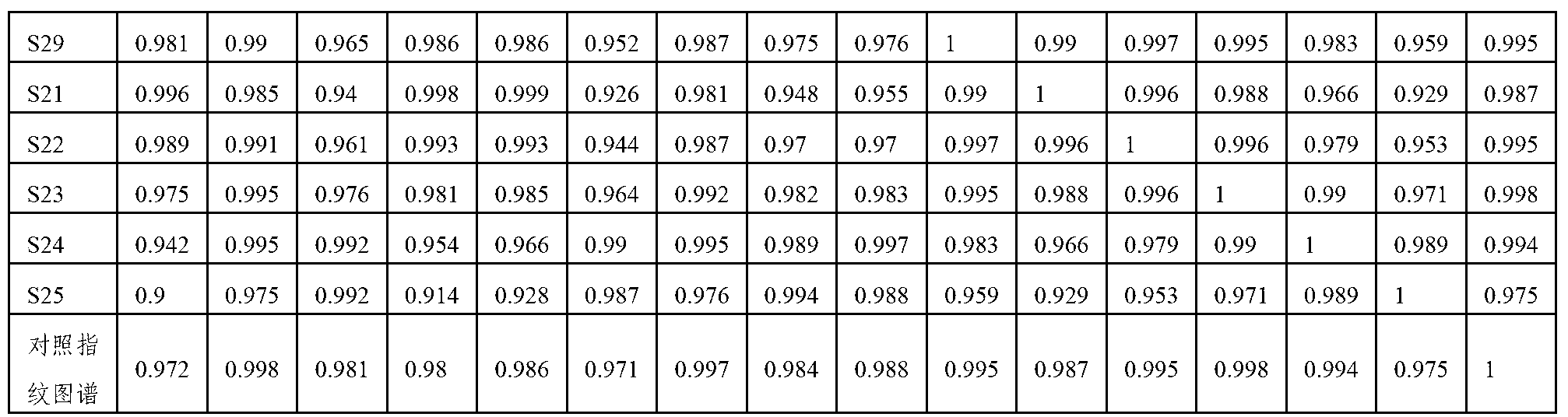
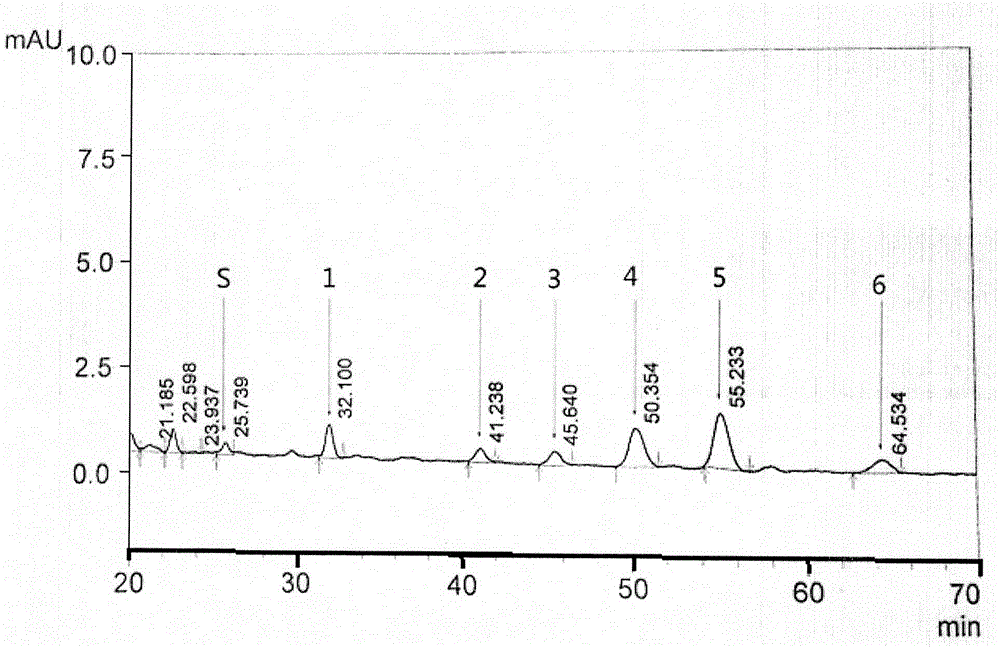
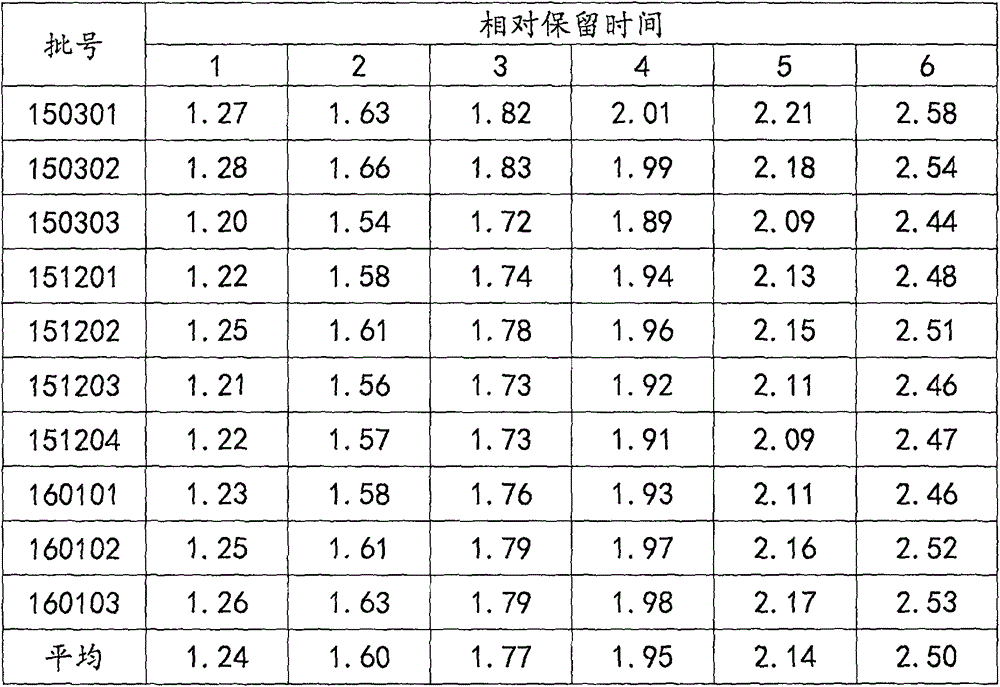
The invention relates to a control method of the fingerprint spectrum quality of cordyceps sinensis powder raw material in botanical drug for strengthening vital qi and removing blood stasis, comprising the steps that: (1) cordyceps sinensis powder is extracted: 0.100g of cordyceps sinensis powder is taken, purified water is added, the ultrasonic extraction, the filtration and the sample injection are carried out; (2) the gradient elution with mobile phase is carried out: octadecyl silane bonded silica gel is taken as a filler, water and acetonitrile are taken as mobile phase to carry out the gradient elution for 0 to 30min and 0 to 7 percent B; (3) a standard fingerprint spectrum is established: the HPLC standard fingerprint spectrum of the cordyceps sinensis powder is determined, and 3 characteristic peaks are selected; (4) the quality control of the fingerprint spectrum is carried out: the relative retention time of No.2 peak uridine, No.3 peak guanosine and No.4 peak adenosine are 0.44 plus or minus 0.03, 0.68 plus or minus 0.03 and 1.00 respectively; the HPLC fingerprint spectrum of the sample is compared with the contrast fingerprint spectrum. The similarity calculated by the 5 common peaks is not less than 0.9, (5) the preparation of the cordyceps sinensis powder raw material is carried out; the control method has good repetitivity and can fully reflect the basic characteristics of nucleoside ingredients of the cordyceps sinensis powder.
Owner:SHANGHAI MODERN CHINESE TRADITIONAL MEDICINE TECH DEV
Method for controlling quality of corydalis tuber and preparation thereof and drug effect thereof by using finger print
InactiveCN101288699AEffective representation qualitySuitable for useComponent separationCardiovascular disorderPalmatineCorydalis cava
The invention relates to a fingerprint map for controlling the quality and efficacy of rhizoma corydalis and preparation of the rhizoma corydalis and a preparation method of the fingerprint map. The invention discloses that the fingerprint map is one of anti-myocardial ischemic active site in the rhizoma corydalis, and the active site mainly comprises quaternary ammonium base components, wherein, columbamine, 13-methyldehydrocorydalmine, dehydrocorybulbine, palmatine, dehydrocorydaline are the main five components of quaternary ammonium base. The fingerprint map can identify the authenticity of samples and evaluate the quality and efficacy of the rhizoma corydalis or the preparation thereof, so that the quality of the rhizoma corydalis and the preparation thereof have real controllable standards, so as to ensure the product quality to be stable. The method has the advantages of being simple to operate and being accurate and reliable, which is applicable to controlling the quality and efficacy of the rhizoma corydalis and the preparation thereof.
Owner:INST OF MEDICINAL PLANT DEV CHINESE ACADEMY OF MEDICAL SCI
Method for identifying medicinal materials using characteristic atlas
InactiveCN1588050AImprove stabilityImprove featuresComponent separationPattern recognitionData acquisition
The invention relates to a identification method for drugs with characteristic graphoes. The method acquire the characteristic graphoes with liquid phase chromaspectrum-mass spectrum combining technology and identify medicine with the characteristic graphoes. The identification method provided by the invention is based on the characteristic graphoes,the steps includes data acquirement, chromatography matching, the data normalization processing,the characteristic selection and the characteristic graphoes establishment. The invention has the following advantages and remarkable effect compared to the current method of adopting the original fingerprint map to identify drugs: 1)the specification of the data acquisition system is low,the characteristic graphoes has good stability, specificity and objectivity; 2)the characteristic graphoes causes the increas of the difference of the sample in the same category and between the different categories, getting better category charateristics, its identifying ability is better than the original fingerprint graphoes; 3)the identification result of the drugs is accurate, available and reliable.
Owner:TSINGHUA UNIV
Pericarpium Trichosanthis or Pericarpium Trichosanthis injection liquid chromatography fingerprint test method
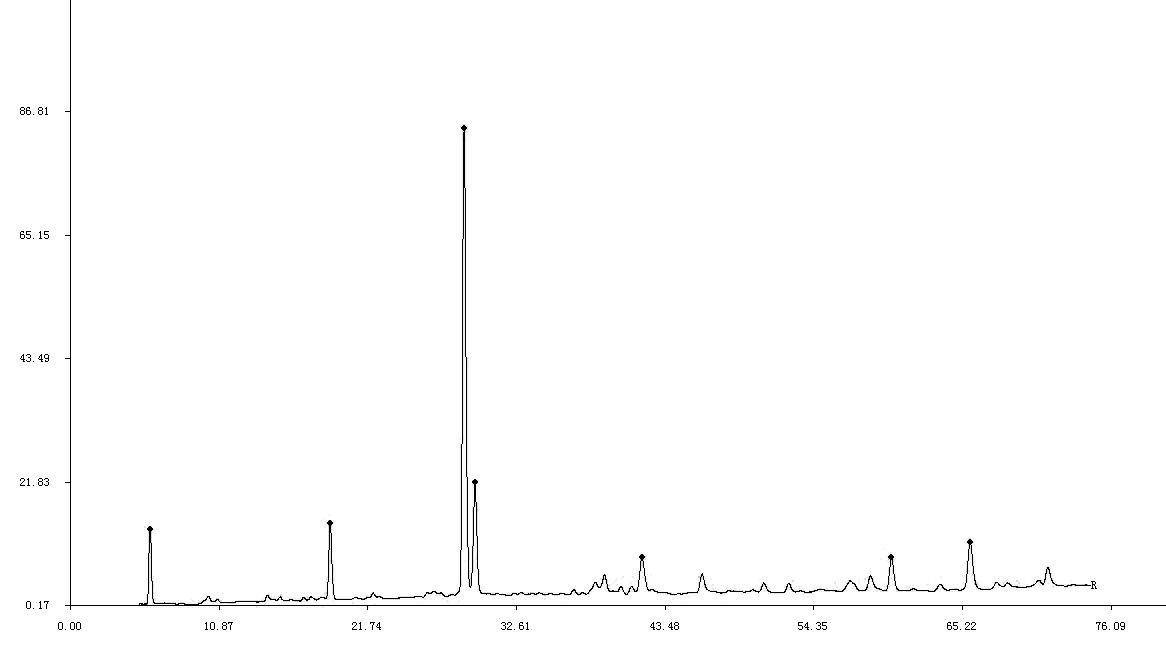
The invention discloses a test method of liquid spectrum finger diagram of melon, or melon injection, comprising that (1), preparing melon, or melon injection into a sample solution, (2), respectively absorbing citrulline water solution and the sample solution, to be filled into a liquid spectrometer, and using the solution of phthalaldehyde and the solution of chloride aminic acid fluorenes methyl ester to process derivatization, (3), testing liquid spetrum. The inventive test method first discloses a liquid spectrum finger diagram of melon or melon injection, with high accuracy, stability and repeatability, as one analysis test method which can control the product quality, used in product quality test. The inventive method first obtains the high-effect liquid spectrum contrast finger diagram of melon and melon injection, as diagram 1 and diagram 2.
Owner:SPH NO 1 BIOCHEM & PHARMA CO LTD
Construction method for ion chromatography fingerprint spectrums of ganoderma lucidum spore powder polysaccharide
ActiveCN102645504AMethod stableHigh precisionComponent separationAnion-exchange chromatographyOligosaccharide
The invention discloses a construction method for ion chromatography fingerprint spectrums of ganoderma lucidum spore powder polysaccharide, which comprises acid-enzyme partial hydrolysis of the ganoderma lucidum spore powder polysaccharide, the ion chromatography fingerprint analysis of monosaccharide components and oligosaccharide components of hydrolysis products of the ganoderma lucidum spore powder polysaccharide and the confirmation of standard fingerprint spectrums. By means of the high performance anion-exchange chromatography (HPAEC) analysis and the comparison of the fingerprint spectrums of polysaccharide contents in ganoderma lucidum spore powder samples from 20 different production places, the common fingerprint characteristics of the polysaccharide contents in the ganoderma lucidum spore powder samples are determined, the standard fingerprint spectrums of the monosaccharide components and the oligosaccharide components are obtained respectively, and 11 monosaccharide common characteristic peaks in monosaccharide spectrums, 4 oligosaccharide common peaks in the oligosaccharide spectrum and 1 polysaccharide peak are determined. According to the construction method for the ion chromatography fingerprint spectrums of the ganoderma lucidum spore powder polysaccharide, the method is stable, the precision degree is high, the reproducibility is good, the method is easy to master, quality conditions and production places of the ganoderma lucidum spore powder polysaccharide can be grasped from two aspects of fingerprint spectrums of the monosaccharide components and the oligosaccharide components of the acid-enzyme partial hydrolysis products which can reflect the components and structural characteristics of the ganoderma lucidum spore powder polysaccharide, and a new scientific method is provided for the quality control and the authenticity identification of the ganoderma lucidum spore powders.
Owner:中食都庆(山东)生物技术有限公司
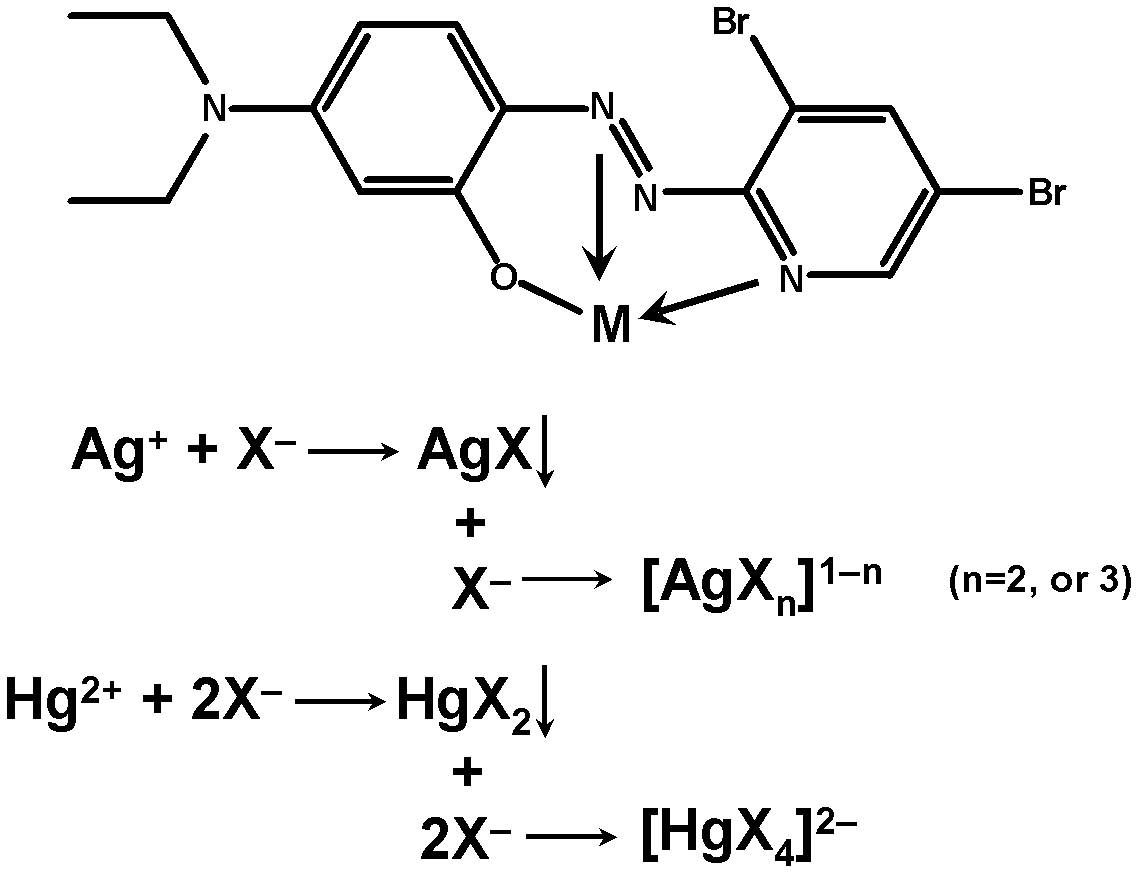
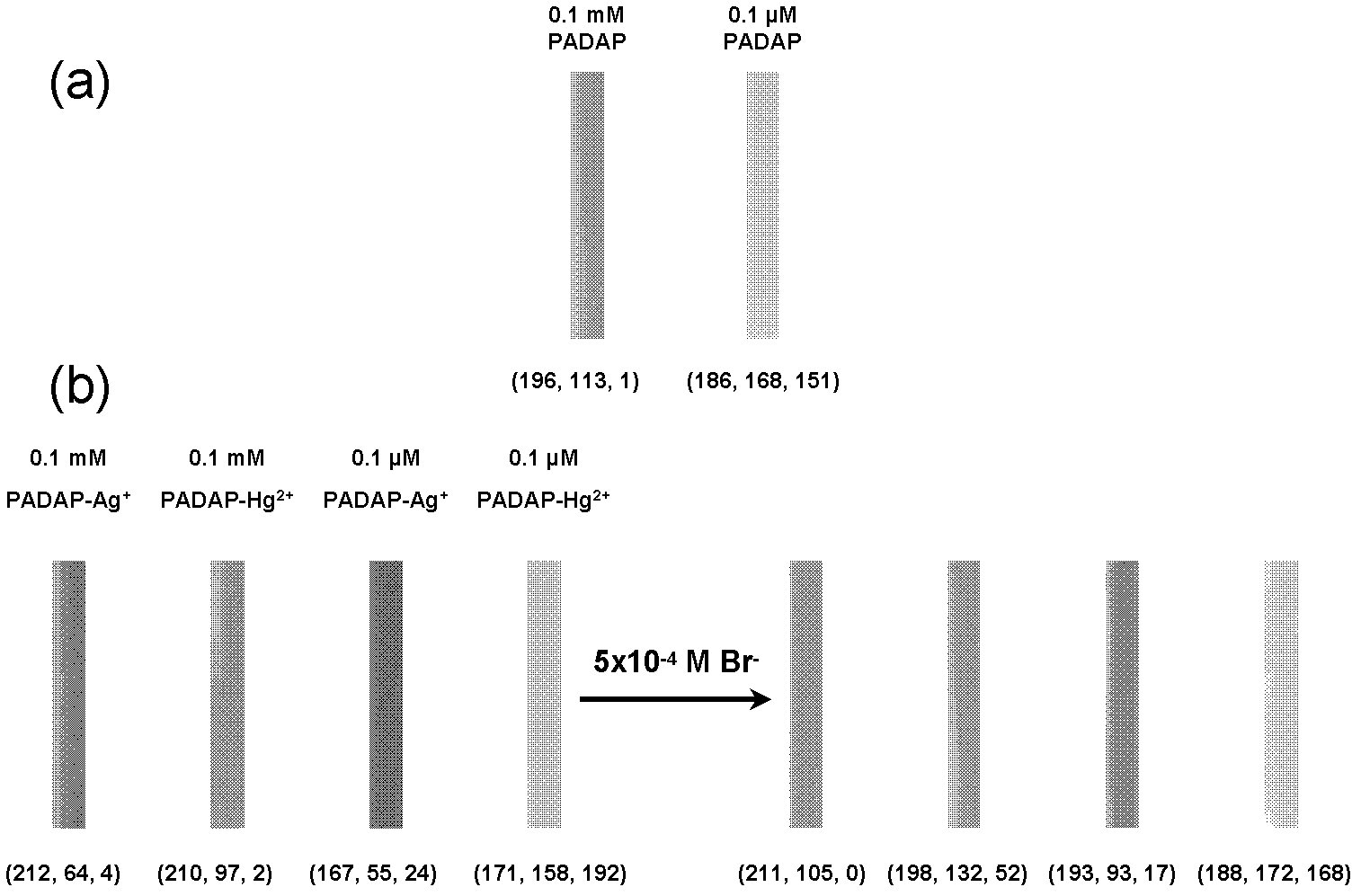
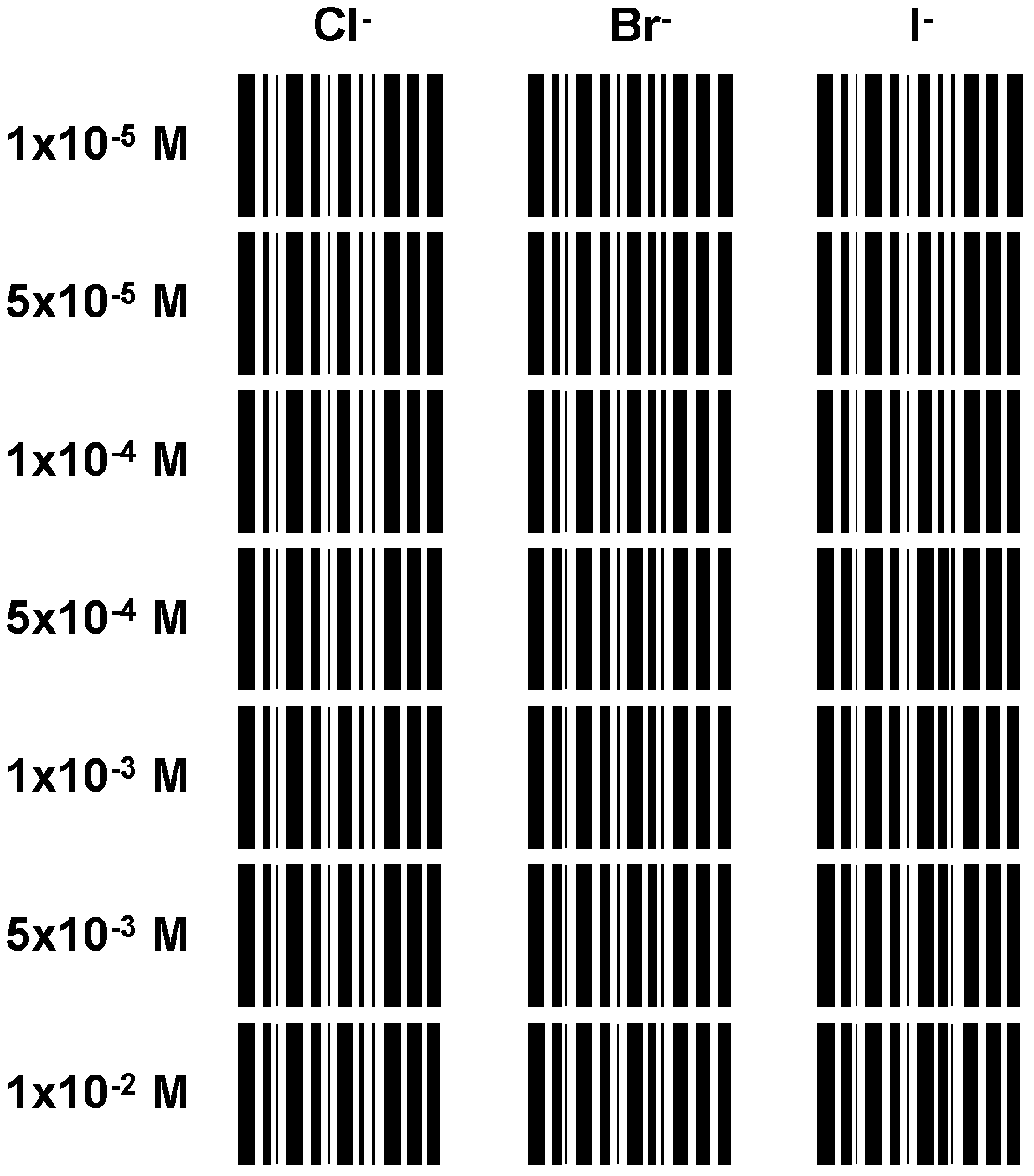
Method for semi-quantitatively determining chloride, bromide and iodine ions by indicator displacement reaction
ActiveCN103018233AGood for statistical analysisAdd color variationMaterial analysis by observing effect on chemical indicatorPrincipal component analysisDisplacement reactions
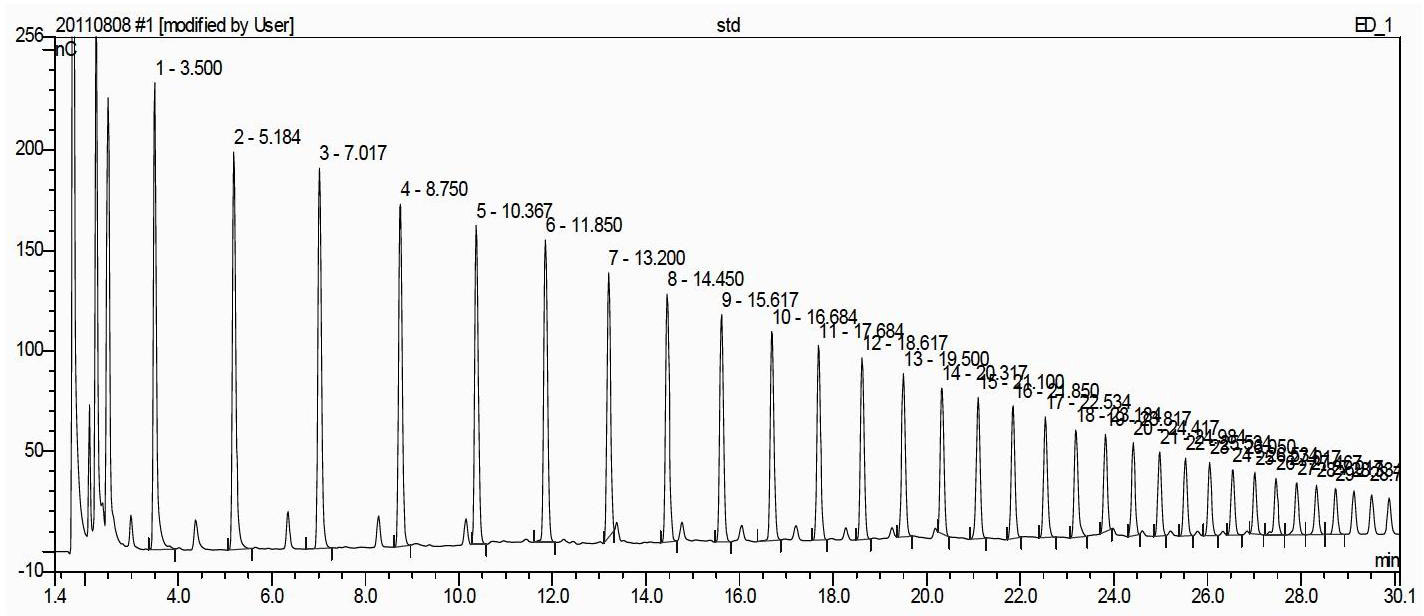
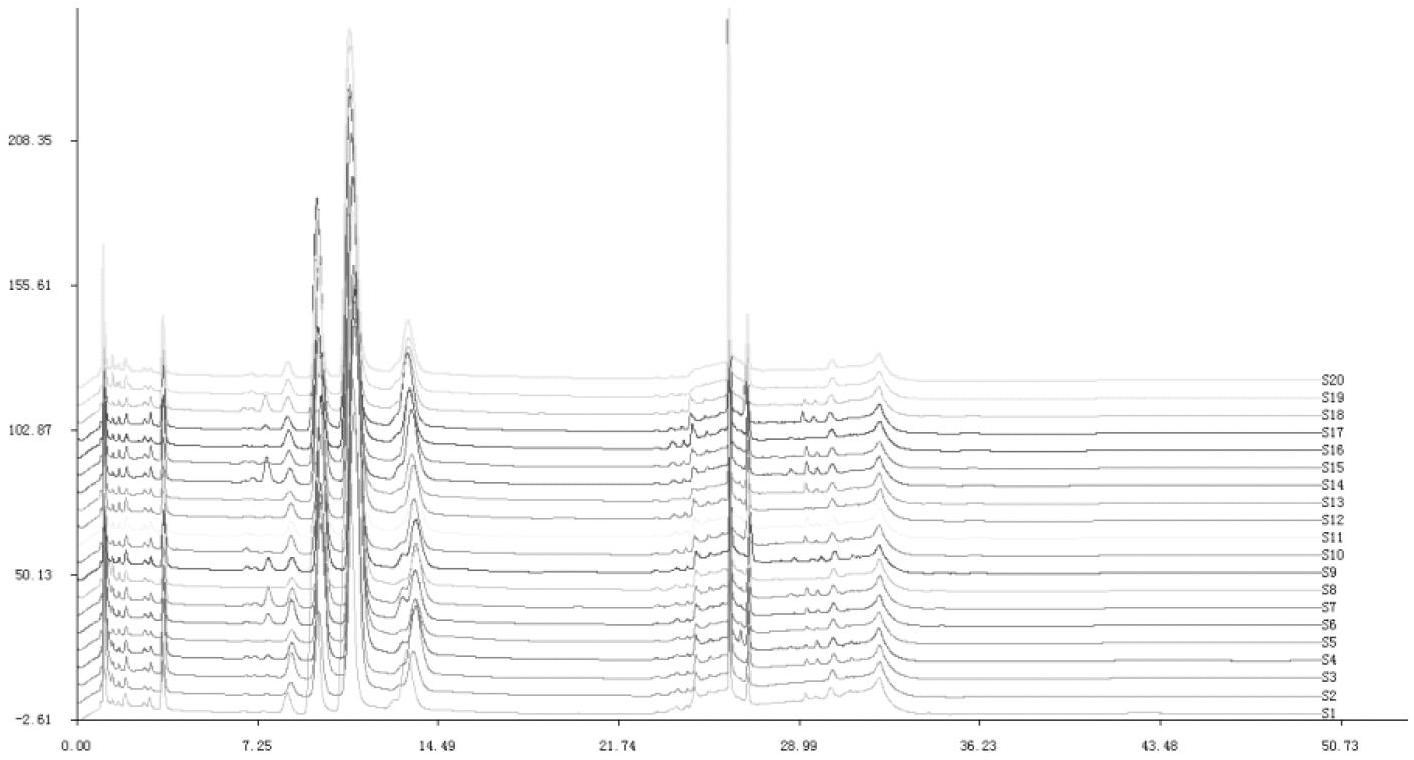
The invention relates to a method for semi-quantitatively determining chloride, bromide and iodine ions by indicator displacement reaction. The method comprises chelating an indicator with a metal ion, and replacing the metal ion from the chelate by chloride, bromide or iodine ions through precipitation reaction of the chloride, bromide or iodine ions to the metal ion, so that the chelate returns to the initial color of the indicator. In the method, color imaging apparatuses (scanner, digital camera, etc.) are used for collecting the color change of the indicator; and a fingerprint spectrum of corresponding concentration of chloride, bromide or iodine ions is constructed according to the red, green and blue spectral data corresponding to the color change. In the sample testing process, a mathematical statistical method, such as clustering analysis or principal component analysis, is adopted to compare the sample spectrum with the fingerprint spectrum database, so as to carry out semi-quantitatively analysis on unknown concentrations of chloride, bromide or iodine ions.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Use of cereour bacillus in preparing thrombus treating medicine
InactiveCN1857722AProlong formation timeObvious antithrombotic effectBacteriaPeptide/protein ingredientsHplc fingerprintDisease
The present invention relates to a kind of streptokinase, and is especially one kind of Bacillus cereus produced fibrinolysin, or ceryl kinase. The ceryl kinase has HPLC fingerprint with peak within 7-9 min in the conditions of chromatographic column Waters Protein -PakTM 60 7.8*300 mm, mobile phase 0.2mol / L NaH2PO4-CH3OH(95:5), flow rate 1ml / min and detecting wavelength 220 nm. The present invention also provides the preparation process of the ceryl kinase, and the use of the Bacillus cereus and the ceryl kinase in preparing thrombus treating medicine. The ceryl kinase of the present invention, the rat carotid artery thrombosis experiment shows, possesses obvious thrombosis resisting effect, so that the present invention provides one new option for treatment of thrombus diseases.
Owner:CHENGDU DIAO JIU HONG PHARMACEUTICAL FACTORY
Method for detecting amino acid component in Xiasangju preparation
ActiveCN1865988AEffectively Characterize QualityMonitor qualityComponent separationPreparing sample for investigationSodium acetrizoateFluorescence
The related detection method for amino acid content in Xiasangju preparation comprises: (a) preparing reference solution; (b) preparing sample solution; (c) chromatogram condition: using octadecylsilicane chemically bonded silica as filler, using the gradient elution liquid with 0.05~0.10mol / L NaAc solution buffer and 20-80% acetonitrile solution as mobile phase with pH value as 4.50-5.05, 30-50Deg temperature, activation wavelength 250nm, emission wavelength 395nm, flow rate as 0.5-1.5mL .min-1, and 30-80min; (d) obtaining the fingerprint spectrum with HPLC. This invention is simple and stable, and has well repeatability and precision.
Owner:GUANGZHOU XINGQUN PHARMA
Gentiana macrophylla capsule fingerprint spectrum and application of spectrum to quality control and component analysis
ActiveCN107796892AGood analysis and evaluation abilityComponent separationHplc fingerprintLoganic acid
The invention relates to a gentiana macrophylla capsule fingerprint spectrum and an application of the spectrum to quality control and component analysis. According to the spectrum, a HPLC (high-performance liquid chromatography) fingerprint spectrum common mode of a gentiana macrophylla capsule is determined by the aid of a traditional Chinese medicine chromatographic fingerprint spectrum similarity evaluation system (2004A), 23 common peaks are calibrated, similarity is 0.966-0.982, and the content of loganic acid, shanzhiside methyl ester, 6'-O-beta-D-glucose gentiopicroside, swertiamarin,gentiopicroside, sweroside, isoorientin and isovitexin in 10 preparations is measured. According to the spectrum, a HPLC fingerprint spectrum method of the gentiana macrophylla capsule and a content measuring method are built, the method has good analysis evaluation ability and the advantages of accuracy, convenience, stability, reliability and the like, so that the method can serve as an effective evaluation method for the quality of the preparation.
Owner:XIAN C P PHARMA
Tibet picrorhiza rhizome composition with specific spectrum effect relationship
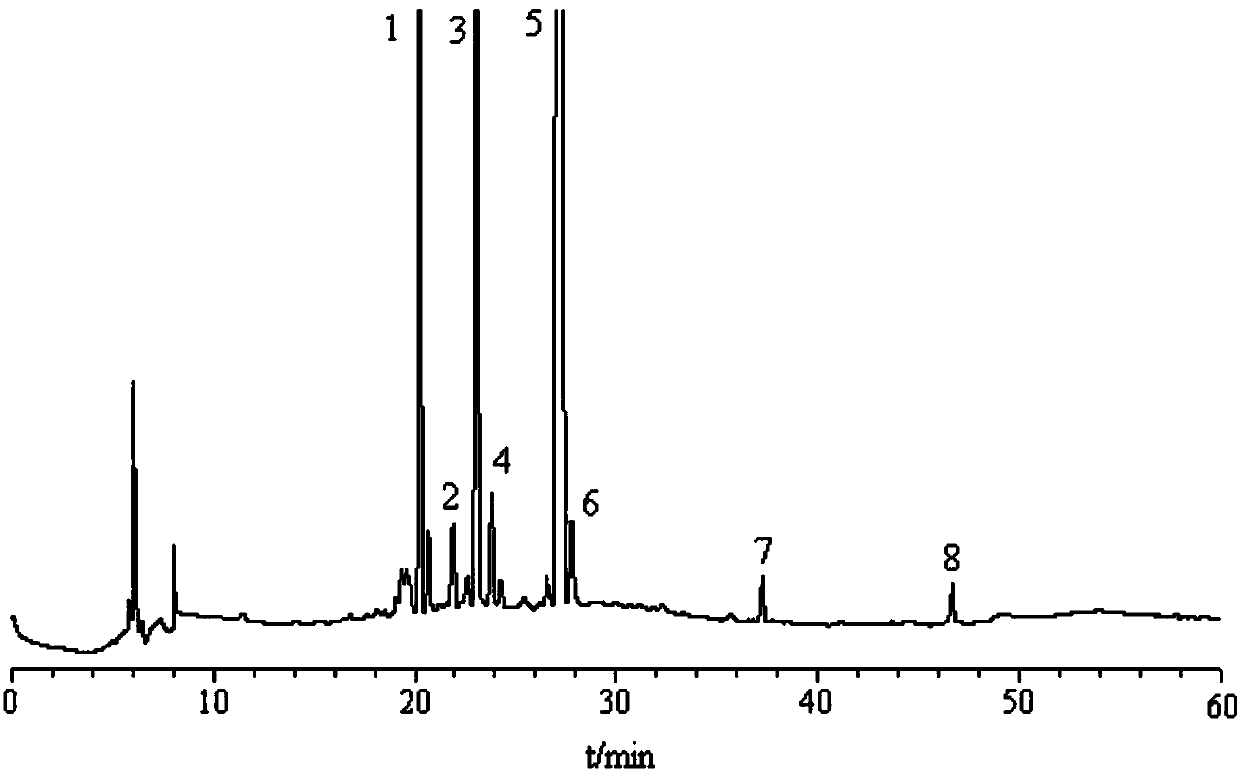
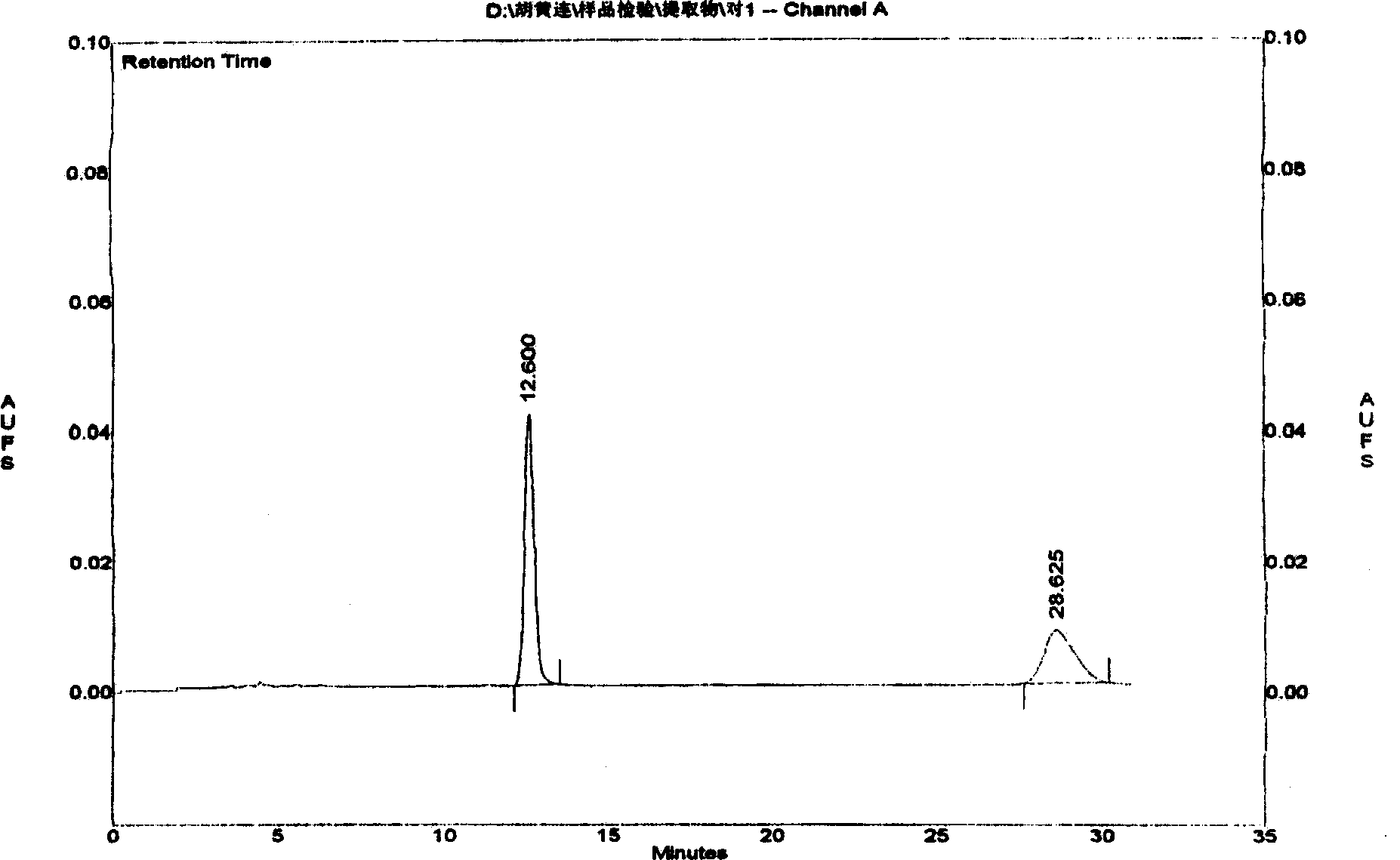
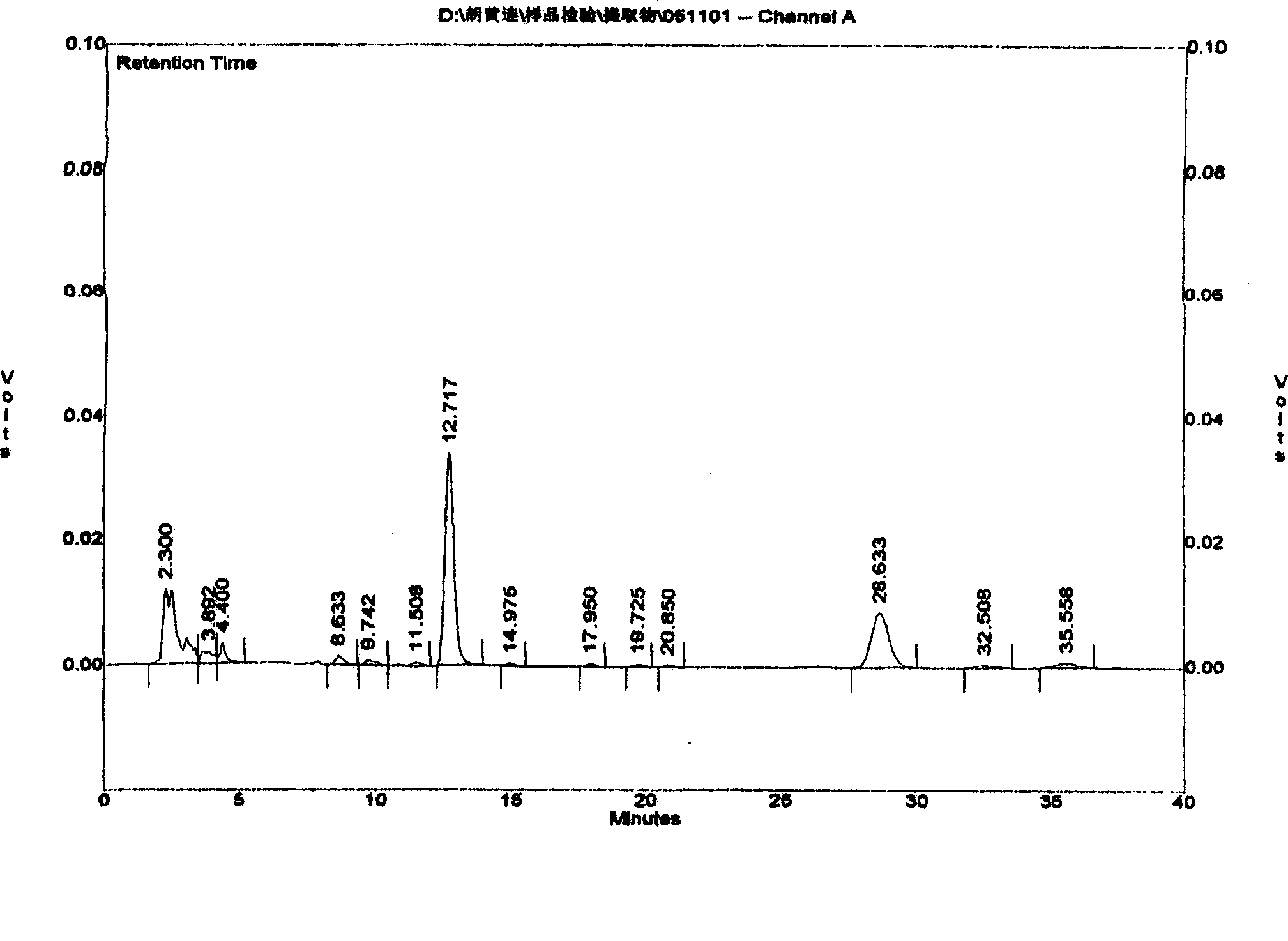
The invention relates to a Tibetan picroside extract with a definite spectrum-activity relationship, in particular to a Tibetan picroside extract contained with picroside I (C24H28O11) and picroside II (C23H28O13), and the sum of the contents of the picroside I (C24H28O11) and the picroside II (C23H28O13) is not lower than 50 percent; and on the fingerprint for the extract, the peak area ratio between the picroside II and the picroside I is 1.30 to 2.40 calculated according to the total picroside chromatogram. The extract has the remarkable action of liver injury prevention and has good application prospect in the field of liver injury prevention medication.
Owner:CHENGDU SINO STRONG PHARMA
Establishing method of rhizoma anemarrhenae HPLC-ELSD (High Performance Liquid Chromatography-Evaporative Light Scattering Detector) fingerprint and standard fingerprint established by using establishing method
InactiveCN103267818AEasy to separateOvercoming separationComponent separationControl substancesXanthone
The invention relates to an establishing method of rhizoma anemarrhenae HPLC-ELSD (High Performance Liquid Chromatography-Evaporative Light Scattering Detector) fingerprint and a standard fingerprint established by using the establishing method. The establishing method comprises chromatographic conditions, the preparation of a test substance solution, and the preparation of a control substance solution, wherein the chromatographic condition is a Thermo C8 chromatographic column (4.6mm*250mm, 5 microns); a mobile phase is an acetonitrile-0.2% acetic acid aqueous solution for gradient elution; the column temperature is 20 DEG C; the flow speed is 1mL.min<-1>; the flow speed of an ELSD atomizer is 2.6L.min<-1>; the temperature of a drift tube is 100 DEG C; and the sampling size is 20 microlitres. The establishing method provided by the invention has the advantages of good stability and repeatability, high precision, capability of simultaneous reflection of the characteristics of saponins and xanthones, which are the main chemical ingredients of the rhizoma anemarrhenae, and capability of reflecting the quality of the rhizoma anemarrhenae according to the overall characteristics of the chromatogram; and the establishing method can be used as one method for controlling the quality of the rhizoma anemarrhenae.
Owner:GUIYANG COLLEGE OF TRADITIONAL CHINESE MEDICINE
A kind of quality detection method of traditional Chinese medicine Eucommia ulmoides preparation
ActiveCN102262132AQuality improvementHigh precisionComponent separationChlorogenic acidBULK ACTIVE INGREDIENT
The invention relates to a method for detecting the quality of a Chinese medicine eucommia preparation and belongs to the technical field of medicine quality control. The method comprises the following steps of: (1) preparing reference substance solution; (2) preparing test sample solution; (3) diluting gradually; (4) drawing a standard fingerprint graph by taking chlorogenic acid as a reference peak; and (5) controlling the quality of the fingerprint graph. Compared with the prior art, the method has the advantages that: the standard fingerprint graph is constructed by adopting efficient liquid phase, and quantified parameters are obtained by taking the characteristics of active ingredients of the eucommia as major through the fingerprint graph; the method is higher in precision and highin stability and repeatability; and the quality of the preparation can be controlled more comprehensively and effectively.
Owner:JIANGXI POZIN PHARMA
Detection method for fingerprint spectrum of medicinal preparation treating hepatitis
InactiveCN105181825AImprove securityImprove stabilityComponent separationDigestive systemChlorogenic acidMedicine
The invention relates to a detection method for a fingerprint spectrum of a medicinal preparation treating hepatitis. In the detection method, through optimization selection of a No.11 peak of baicalin as an internal reference peak of a fingerprint spectrum, retention time of characteristic common peaks: a No.3 peak of chlorogenic acid, a No.4 peak of gentiopicroside, a No.5 peak of paeoniflorin, a No.6 peak of liquiritin, a No.8 peak of ferulic acid and the like of a yindan pinggan capsule is determined, the yindan pinggan capsule can be detected comprehensively and rapidly, which facilitates comprehensive quality detection and whole quality control, and therefore safety and stability of medicine usage is raised.
Owner:ZHANGZHOU PIEN TZE HUANG PHARM
Methods for establishing fingerprints of cistanche extracts and identifying quality of drugs
InactiveCN101701948ASignificant advantagesSignificant useComponent separationPlant ingredientsHplc fingerprintMedicine
The invention provides methods for establishing fingerprints of cistanche extracts and identifying quality of drugs, comprising preparation of test solution, facture of fingerprints, determination of standard fingerprints and identification of cistanche drugs. HPLC fingerprints are established for methanol extracts of the cistanche drugs with different places of production, different quality or different storage time, and 21 common characteristic peaks are determined through analysis and comparison and form the fingerprint characteristics of the cistanche drugs, and the fingerprint characteristics can be used as the standard fingerprints of the cistanche drugs. The fingerprints of the cistanche drugs needing to be identified can be compared with the standard fingerprints and the common characteristic peaks are detected to identify the quality of the cistanche drugs. The methods are characterized by simpleness and convenience, good reproducibility, multiple characteristic peaks, accuracy, reliability and the like, and ensure the quality control of the cistanche drugs to be more perfect and scientific.
Owner:BIOCHEM ENG COLLEGE OF BEIJING UNION UNIV
UPLC method for detecting components in radix puerariae, radix puerariae extract and radix puerariae-containing preparation
ActiveCN106370763AAccurate quality controlShorten the timeComponent separationMedicinal herbsPuerarin
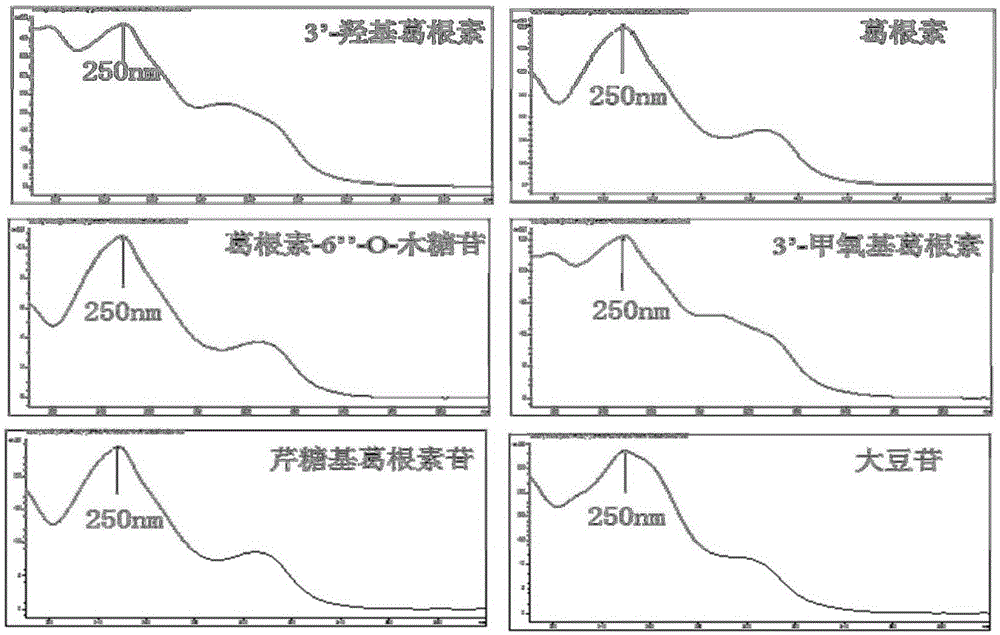
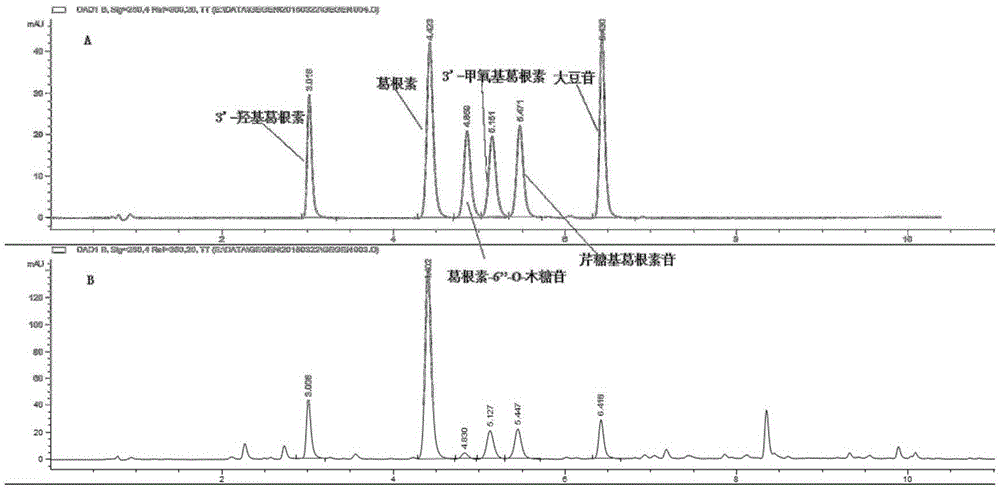
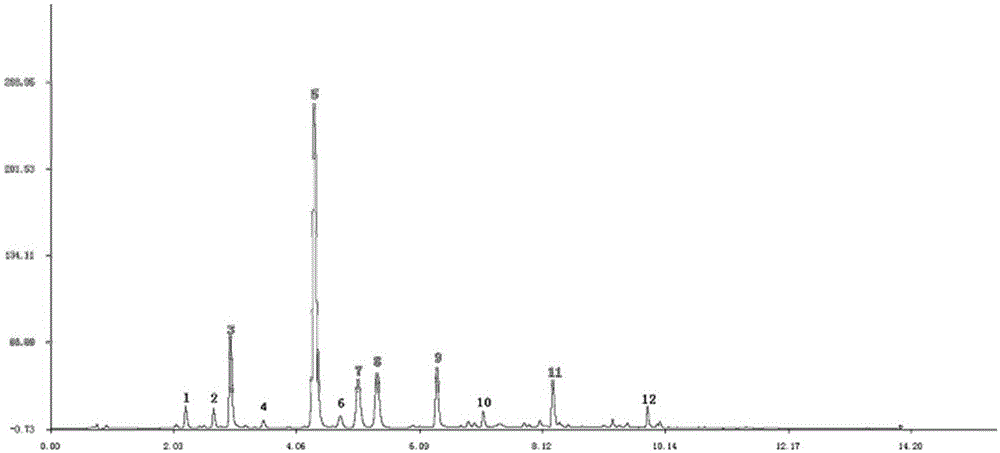
The present invention provides a UPLC method for qualitatively and quantitatively detecting the contents of six components such as 3'-hydroxy puerarin, puerarin, puerarin-6"-O-xyloside, 3'-methoxy puerarin, mirificin and daidzin in radix puerariae, radix puerariae extracts and radix puerariae-containing preparations by adopting puerarin as a reference substance. The UPLC method comprises: preparing a reference substance solution and a testing sample solution, and carrying out gradient elution by adopting 0.1% acetic acid as a mobile phase A and adopting acetonitrile as a mobile phase B, wherein the detection wavelength is 250 nm, the chromatographic column is SB-C18, RRHD 1.8 [mu]m, 2.1*100 mm, the column temperature is 31 DEG C, the flow rate is 0.4 mL / min, and the injection volume is 1 [mu]L; comparing the obtained chromatogram with a radix puerariae herb finger print, wherein the main peak is puerarin, and other components are corresponding to various peaks in a one-to-one manner so as to identify the herb; and calculating the contents of the six components in the radix puerariae by combining relative calibration factors (the relative calibration factor of 3'-hydroxy puerarin is 1.253, the relative calibration factor of puerarin is 1.000, the relative calibration factor of puerarin-6"-O-xyloside is 1.422, the relative calibration factor of 3'-methoxy puerarin is 1.297, the relative calibration factor of mirificin is 1.332, and the relative calibration factor of daidzin is 1.030) and the peak area of puerarin in the contrast solution.
Owner:HUAZHONG UNIV OF SCI & TECH
Identification method of paris polyphylla
InactiveCN107102015AColor/spectral properties measurementsMaterial analysis using radiation diffractionMedicinal herbsAlgorithm
The invention provides a single and composite identification method of paris polyphylla. The method is realized by using infrared wide spectrum detection. By the method, a genuine product or a counterfeit product of the paris polyphylla can be identified and distinguished through comprising the similarity degree of the standard fingerprint spectrum with the fingerprint spectrum of medicinal materials to be inspected; the novel method is provided for the identification of the paris polyphylla.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
Method for detecting quality of south isatis root granules
The invention discloses a method for detecting the quality of south isatis root granules and belongs to the technical field of quality control of medicines. The method comprises the following steps of: preparing a comparison product solution; preparing a test article solution; performing gradient elution; making a standard fingerprint spectrum by using quinazoline dione as a reference peak; and controlling the quality of the fingerprint spectrum. In the method, quinazoline alkaloid is selected as an index for controlling the quality of south isatis root; the quinazoline alkaloid in south isatis root crude drugs has anti-toxoid and bacteriostatic actions; and the quinazoline alkaloid is relatively stable and small in content change in the south isatis root crude drugs, a preparation process and a preparation finished product. The standard fingerprint spectrum is established by using a high-efficiency liquid phase, the characteristics of active constituents of the south isatis root are taken as the principle things, and quantization parameters are obtained by using the fingerprint spectrum. The invention has the advantages that: the method is higher in accuracy and good in stabilityand repeatability; and the quality of a preparation can be more comprehensively and effectively controlled.
Owner:JIANGXI POZIN PHARMA
SSR (Simple Sequence repeats) primer group based on Fraxinus Velutina Torr. transcriptome sequencing information development and application of primer group in germplasm identification
InactiveCN104046697APolymorphism richGood repeatabilityMicrobiological testing/measurementDNA/RNA fragmentationAgricultural scienceNucleotide
The invention discloses an SSR (Simple Sequence repeats) primer group based on Fraxinus Velutina Torr. transcriptome sequencing information development and an application of the primer group in germplasm identification, belonging to the technical field of molecular biology. The SSR primer group comprises 42 pairs of primers, wherein the nucleotide sequences of the first pair to the forty-second pair of primers are shown in SEQ ID No.1 to SEQ ID No.84. Experiments verify that the SSR primer group disclosed by the invention has the advantages of rich polymorphism, good repeatability, clear electrophoretic band and the like. The SSR primer group can be effectively used for researches on germplasm identification, DNA fingerprint establishment and the like. A germplasm identification method is sensitive and reliable, can be used for rapidly and accurately realizing and finishing distinguishment and identification of Fraxinus chinensis germplasm, and has important significances in identification and intellectual property protection of Fraxinus chinensis germplasm resources and genetic breeding of genetic breeding molecules.
Owner:CHINA AGRI UNIV +1
Folium artemisiae argyi extract extracted by ethyl acetate and preparation and detection methods and application thereof
The invention relates to folium artemisiae argyi extracts, methods and application and particularly relates to a folium artemisiae argyi extract extracted by ethyl acetate and preparation and detection methods and application thereof. Effective ingredients of the folium artemisiae argyi extract consist of flavonoids and sterols. The invention provides application of the folium artemisiae argyi extract in the preparation of anti-tumor drugs, and provides an analysis method for constructing a folium artemisiae argyi extract fingerprint by using an ultrahigh performance liquid chromatography-mass spectrometry (UPLC-MS) analysis technology and analysis results. The method comprises the step of acquiring the UPLC-MS analysis conditions for the folium artemisiae argyi extract, features of chromatographic peaks, compound structure information provided by mass spectrums of chromatographic components, and the like. The fingerprint and technology thereof disclosed by the invention can be applied to the species identification of folium artemisiae argyi and the quality monitoring on the folium artemisiae argyi extracts.
Owner:INST OF MEDICINAL PLANT DEV CHINESE ACADEMY OF MEDICAL SCI +1
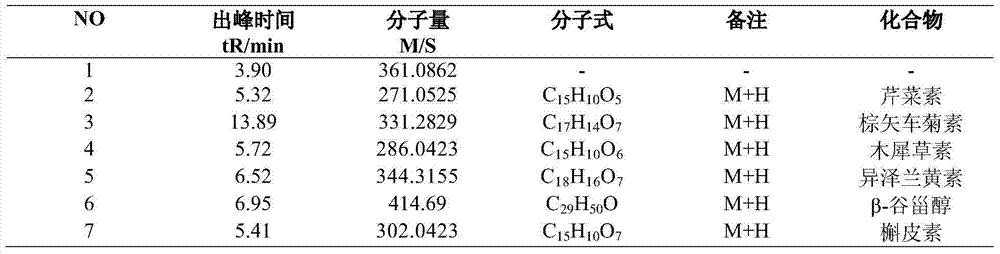
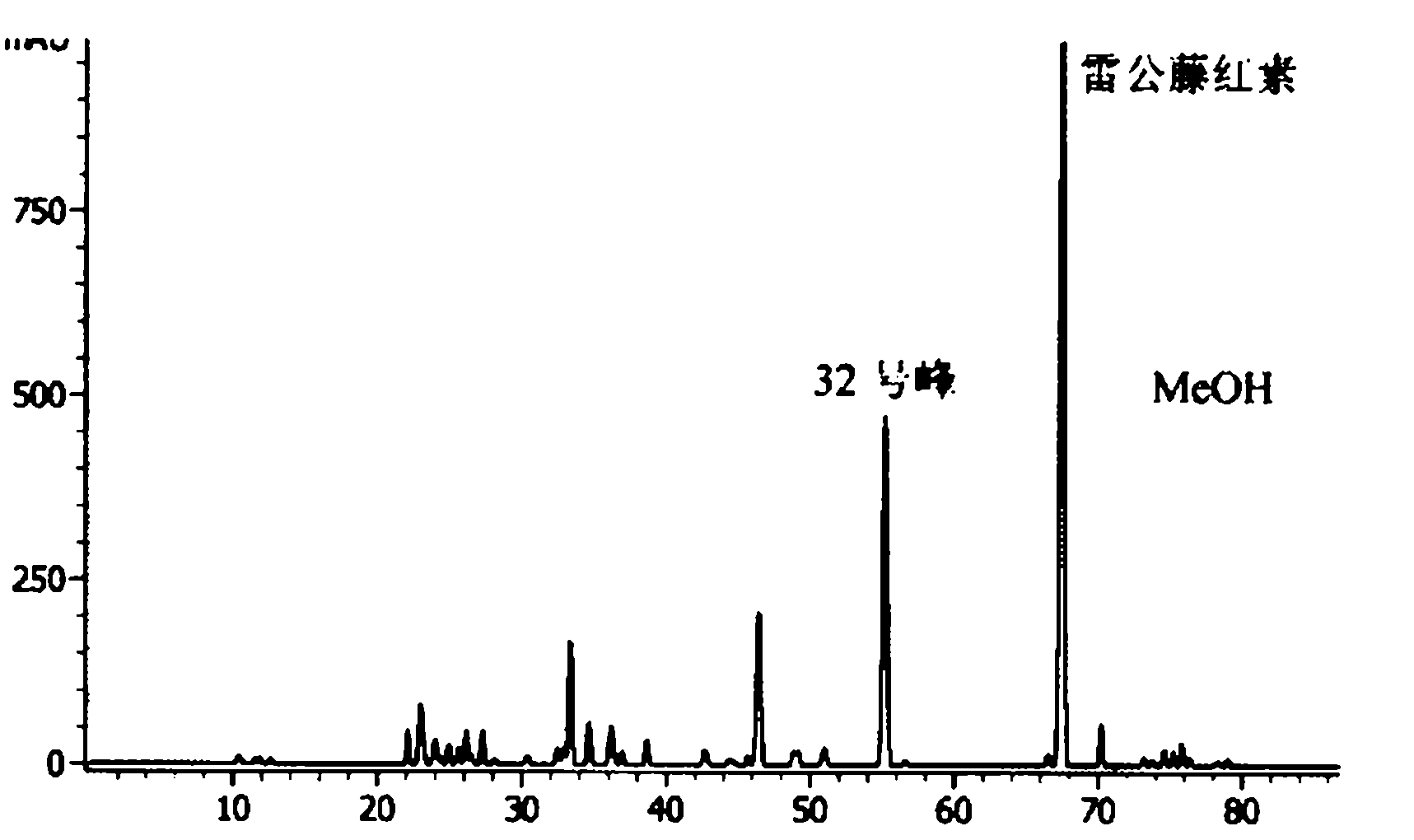
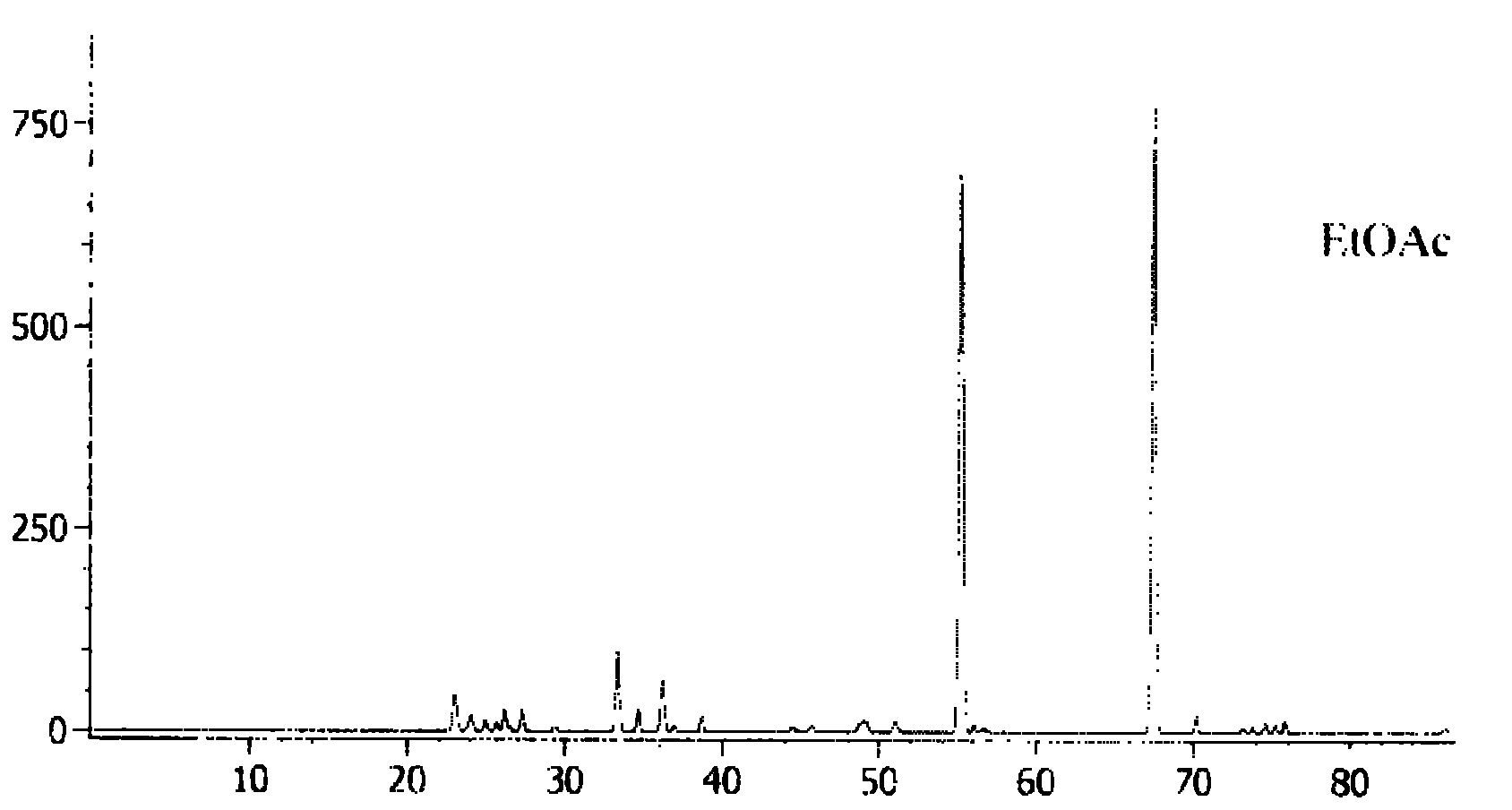
Quality detection method for tripterygii hypoglauci medicinal materials
The invention aims to disclose a quality detection method for a fingerprint spectrum of tripterygii hypoglauci medicinal materials. An acetonitrile-methyl alcohol-0.02% of phosphoric acid solution is used as a moving phase; gradient elution is carried out; and the injection temperature is 20 DEG C. With the adoption of the fingerprint spectrum provided by the invention, not only can the content of triptolide, tripterine and wilforlide be determined, but also the microcosmic components of the tripterygii hypoglauci medicinal materials are analyzed, and a common mode of the fingerprint spectrum is formulated, so that the most advanced level can be reached in qualitative and quantitative aspects.
Owner:GUANGZHOU CHEN LI JI PHARMA FACTORY
Method for preparing siwu decoction formula granules and quality control method thereof
ActiveCN105287875AEmbody conceptTo achieve the purpose of reducing toxicity and increasing efficiencyComponent separationMaterial analysis by optical meansBiotechnologyFormulary
The invention discloses a method for preparing siwu decoction formula granules and a quality control method thereof. The method for preparing the siwu decoction formula granules comprises steps: prepared rehmannia roots, angelica sinensis, radix paeoniae alba and ligusticum wallichii medicinal slices are added with water which is 5-15 times of the weight of total inventory, are extracted for two times, aromatic water is collected and is combined with two frying filter liquids, are decompressed and concentrated in vacuum, a concentrated solution is added with beta (Beta)-cyclodextrin and silicon dioxide to uniformly stir and obtains a clear paste, and the aromatic water and the aromatic water are sprayed and dried after being uniformly mixed, and are pelletized through a dry method to prepare the siwu decoction formula granules. The quality control method of the siwu decoction formula granules comprises qualitative identification of an infrared fingerprint spectrum and a thin layer and content determination of a high performance liquid chromatography (HPLC). The method for preparing siwu decoction formula granules and the quality control method thereof decoct in a combined mode according to a traditional method, can perfectly take full advantages of drug matching compared with an existing method that various medicinal odours are added when single formula particles are taken, reflects the overall concept of Chinese medicine, guarantees to achieve the purpose of reducing toxicity and enhancing efficacy, supplies novel selection for clinical medication, builds a perfect quality standard, controls quality by combining a power-spectral method and a chromatography, and can effectively control quality of complex granules from the overall to the more specific.
Owner:GUANGDONG YIFANG PHARMA
Method for constructing hepatoma protein group fingerprint model and serum detection application thereof
InactiveCN1654953AReduce fatality rateHigh cure rateSamplingComponent separationProtein insertionMortality rate
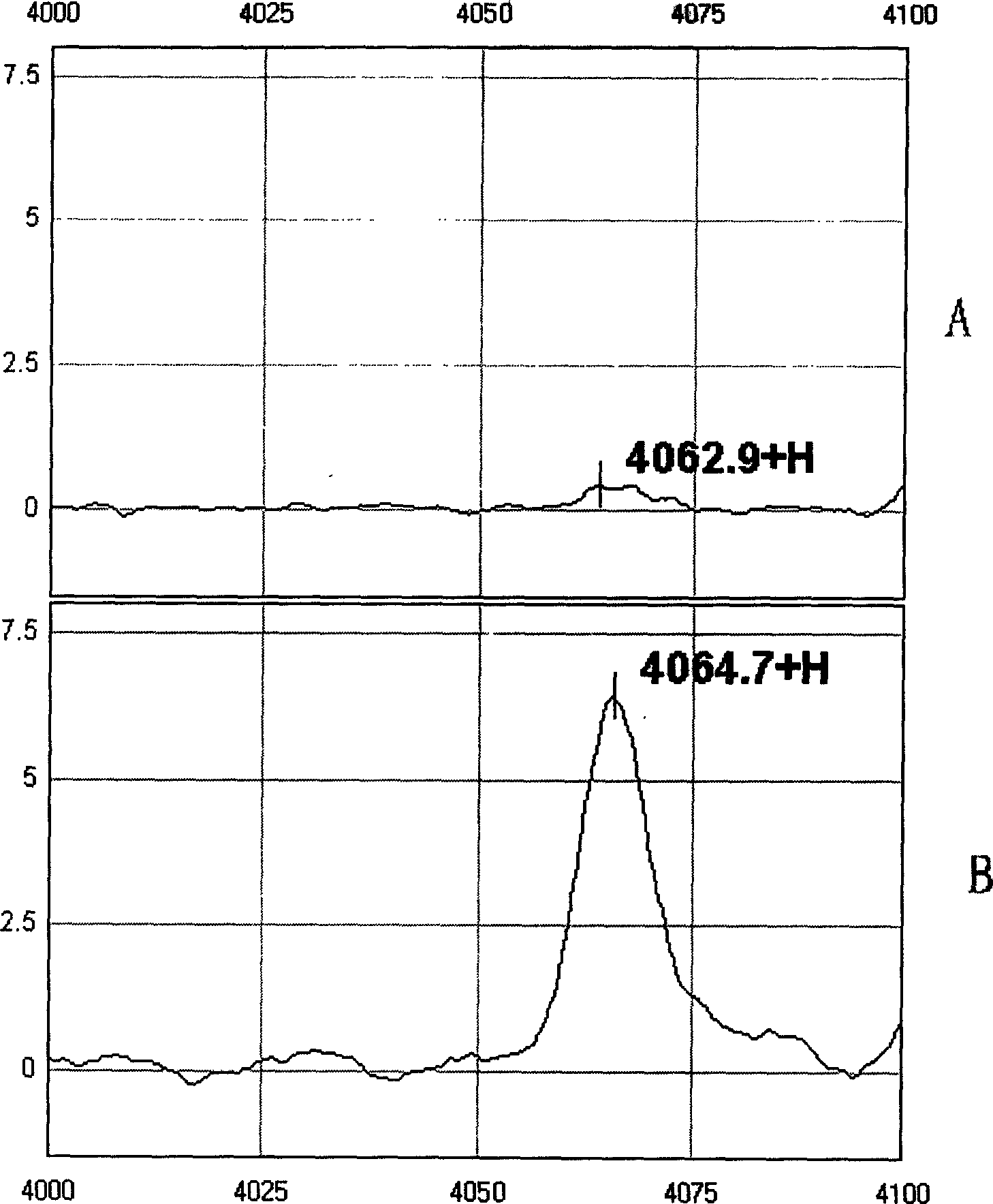
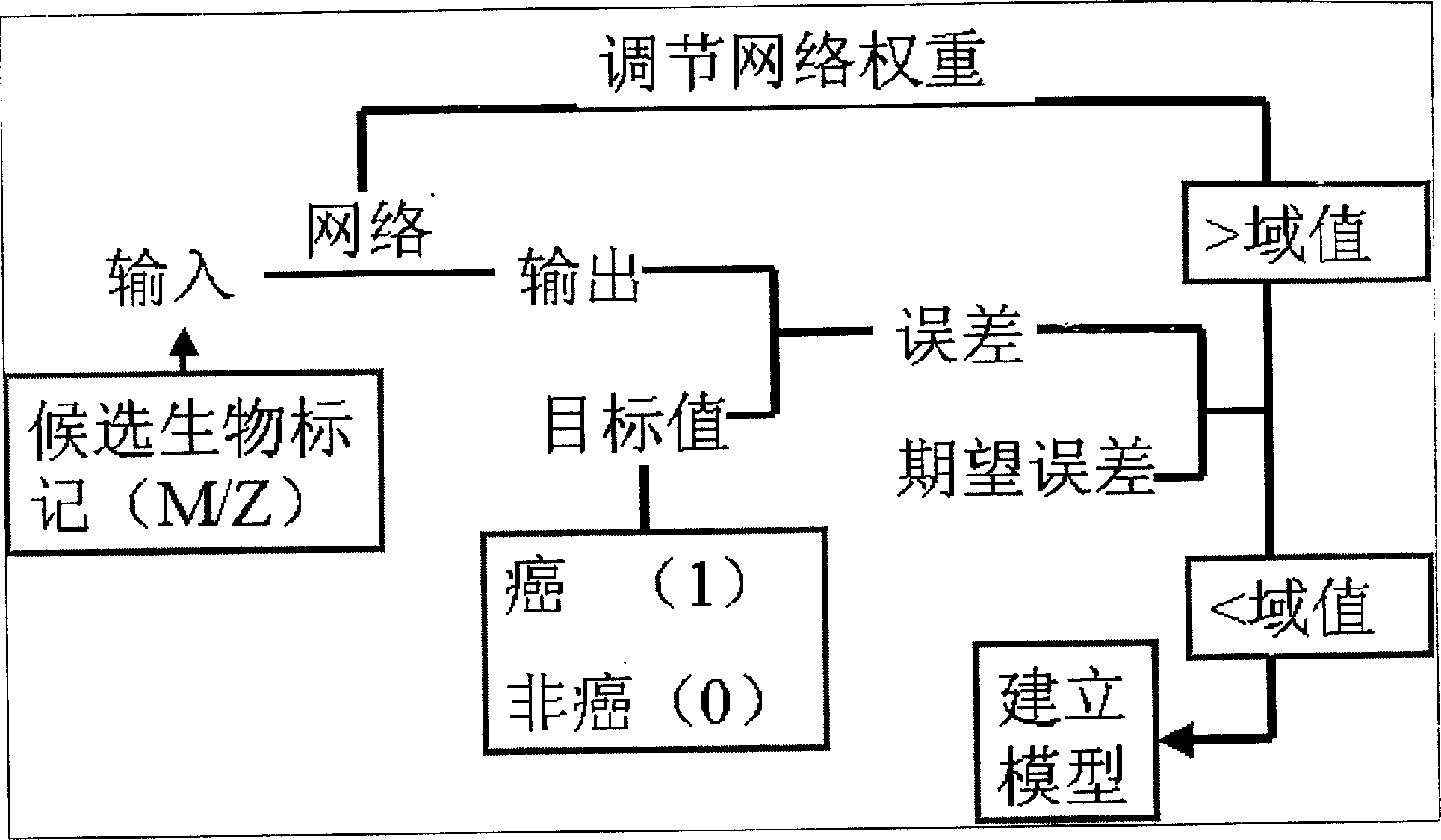
This invention provides a non-invasion for testing liver cancer, which sets up a liver cancer protein set fingerprint atlas model used in the serum test. Said model is composed of a HAS set mass spectrum atlas and an artificial nerve net analysis method used in early serology test. The atlas is composed of the protein with five M / Z. The method for setting up the atlas model includes: 1, preparation of serum, 2, mass spectrum test and collection of data for the serum specimen, 3, artificial nerve net analysis of the spectrum data, which can test the liver cancer before there is not any pathologic change appearing to the cell.
Owner:郭爱林
Fingerprint spectrum detection method for low-sugar intensified loquat distillate
InactiveCN106770719AOvercoming the difficulty of reflecting the real feeding situation of the productQuantitative authenticityComponent separationQuality levelSugar
The invention provides a fingerprint spectrum detection method for a low-sugar intensified loquat distillate, relating to the technical field of drug detection. The fingerprint spectrum detection method comprises the following steps: (1) establishing a component standard characteristic spectrum of the low-sugar intensified loquat distillate; (2) determining a sample characteristic spectrum of a to-be-detected low-sugar intensified loquat distillate by virtue of the same method; and (3) making comparison on the sample characteristic spectrum of the to-be-detected low-sugar intensified loquat distillate and a standard characteristic spectrum of the low-sugar intensified loquat distillate, and judging the quality and authenticity of the low-sugar intensified loquat distillate. The fingerprint spectrum detection method has the beneficial effects that the integrality, the macroscopic characteristic and the fuzziness are realized; the problem that the real adding situation of a product is difficulty reflected by the content determination of a single component is overcome; a novel method and measures are provided for the complete and accurate evaluation of the quality of the low-sugar intensified loquat distillate; the similarity and difference of spectra are visually identified; and the authenticity and quality level of the sample are quantized by virtue of semi-quantitative indexes.
Owner:HEFEI JINYUE PHARMA
Method for creating HPLC (high-performance liquid chromatography) fingerprint spectra of vinegar-processed rhizoma cyperi
The invention provides a method for creating HPLC (high-performance liquid chromatography) fingerprint spectra of vinegar-processed rhizoma cyperi. The method includes extracting vinegar-processed rhizoma cyperi decoction piece powder by the aid of methanol to obtain test solution; utilizing alpha-cyperone, rhizoma cyperi ketene and 5-hydroxymethylfurfural as reference substances; measuring more than 10 batches of vinegar-processed rhizoma cyperi and carrying out analysis and comparison to obtain the HPLC fingerprint spectra of the vinegar-processed rhizoma cyperi. Each HPLC fingerprint spectrum comprises 12 common characteristic peaks. The method for creating the fingerprint spectra has the advantages that the method is excellent in precision, repeatability and stability; the quality of vinegar-processed rhizoma cyperi decoction pieces can be effectively integrally described and evaluated by the aid of the availability of common peaks in the fingerprint spectra, and accordingly the stability of the quality of the detection pieces and the effectiveness and the safety of clinical medication can be guaranteed.
Owner:SHANDONG UNIV OF TRADITIONAL CHINESE MEDICINE
Nymphaea tetragona DNA fingerprint, primer to acquire same and construction method of Nymphaea tetragona DNA fingerprint
PendingCN110129472AHigh polymorphismGood repeatabilityMicrobiological testing/measurementDNA/RNA fragmentationFingerprintMolecular mapping
The invention relates to Nymphaea tetragona DNA fingerprint, a primer to acquire the same and a construction method of the Nymphaea tetragona DNA fingerprint and belongs to the technical field of biology. The construction method of the Nymphaea tetragona DNA fingerprint comprises the specific steps of 1) extracting Nymphaea tetragona DNA; 2) performing ISSR-PCR (inter-simple sequence repeat-polymerase chain reaction) amplification reaction and electrophoresis detection, to be specific, amplifying Nymphaea tetragona DNA with an ISSR primer, and electrophoretically detecting the ISSR-PCR productwith 1.8% agarose gel, wherein the ISSR primer is from one or more of UBC840, UBC841, UBC811, UBC834, UBC835, UBC843, UBC844, UBC845, UBC873, and UBC880; 3) constructing DNA fingerprint. A stripe amplified with a Nymphaea tetragona ISSR-PCR system established herein has good repeatability and high polymorphism; the defects of domestic studies on Nymphaea tetragona germplasm resource genetic diversity and molecular mapping are overcome. The Nymphaea tetragona DNA fingerprint constructed via the method herein can act as basis to identify and classify corresponding primary germplasm resources ofNymphaea tetragona, and also guarantee the classification and identification of new germplasms and new varieties and the protection of intellectual properties at molecular technical level.
Owner:FLOWER RES INST GUANGXI ACADEMY OF AGRI SCI
Fingerprint spectrum detection method of medicinal preparation
PendingCN111999395AImprove quality controlImprove detection efficiencyComponent separationSalvianolic acid BPharmaceutical formulation
The invention provides a fingerprint spectrum detection method of a medicinal preparation. According to the detection method, an HPLC-DAD wavelength switching method is adopted to simultaneously determine a plurality of effective components such as mulberroside A, hydroxysafflor yellow A, paeoniflorin, ferulic acid, calycosin-7-glucoside, rosmarinic acid, salvianolic acid B, formononetin and the like in the medicinal preparation; the sensitivity and the accuracy of the detection method are greatly enhanced, so that the comprehensiveness of quality evaluation of the medicinal preparation is guaranteed.
Owner:SHAANXI BUCHANG PHARMA
Method for detecting fingerprint spectrum of Shengbai Koufuye
The invention discloses a method for establishing a control fingerprint spectrum of Shengbai Koufuye, which comprises the following steps that 1, a test solution is prepared, namely, multiple batchesof qualified Shengbai Koufuye are put into a volumetric flask and the volume is fixed; 2, a reference substance solution is prepared, a reference substance icariin is taken, and the volume is fixed; 3, chromatographic conditions are that octadecylsilane chemically bonded silica is taken as a filler, acetonitrile is taken as a mobile phase A, formic acid water is taken as a mobile phase B, gradientelution of the mobile phase is carried out, the detection wavelength ranges from 200 nm to 300 nm, the column temperature is 25-35 DEG C, the flow velocity is 0.9 ml / min to 1.1 ml / min, and the numberof theoretical plates is not less than 10000 calculated according to icariin peaks; 4, in the determination method, the reference substance solution and the test solution are respectively absorbed, and the solutions are injected into an ultra-high performance liquid chromatograph for determination to obtain a chromatogram; and step 5, common peak confirmation is conducted: the chromatograms of the multiple batches of qualified drugs are processed through a computer model to form a unified chromatogram, and the chromatographic peak with the peak area accounting for more than or equal to 0.8% of the total peak area, good peak pattern and high separation degree is selected as a common peak.
Owner:HUBEI MONYAN PHARMA CO LTD
Establishment method of baphicacanthus cusia HPLC fingerprint
ActiveCN104133003ASignificant advantagesSignificant useComponent separationHplc fingerprintMedicinal herbs
The invention provides an establishment method of a baphicacanthus cusia HPLC fingerprint. The method includes following steps: preparing a to-be-tested sample solution, determining chromatographic conditions, and performing detection with an HPLC instrument to obtain a HPLC fingerprint of a baphicacanthus cusia medicine and extracts thereof. Overall chemical information of the baphicacanthus cusia can be comprehensively expressed and fingerprint characteristics of the baphicacanthus cusia can be determined. The fingerprint is simple in method, is good in repeatability, is many in characteristic peaks and is accurate and reliable. An established HPLC fingerprint can effectively controls quality of the baphicacanthus cusia medicine.
Owner:贵州中医药大学
Cold-coagulation-blood-stasis-syndrome-resistant differential metabolite metabolic pathway and study method of Chinese angelica-based cold-coagulation blood-stasis treatment decoction
InactiveCN110794074AHigh diagnostic valueEfficient revealComponent separationEndogenous metabolismDisease
The invention discloses a cold-coagulation-blood-stasis-syndrome-resistant differential metabolite metabolic pathway and study method of a Chinese angelica-based cold-coagulation blood-stasis treatment decoction. The method comprises: detecting and analyzing endogenous metabolites changed at different time points after cold-coagulation blood-stasis symptoms forming of a female rat under an ice-water bath and epinephrine induction and Chinese angelica-based cold-coagulation blood-stasis treatment decoction intervention by using an ultra-high performance liquid chromatography tandem mass spectrometry so as to obtain a fingerprint spectrum; with a multivariate variable statistical analysis method, carrying out screening from a plurality of variables and identifying 21 significantly changed metabolites; and enriching eight metabolic pathways related to different development stages of the cold-coagulation blood-stasis symptom by using ametaboanalyst open-source online metabonomics analysiswebsite. According to the invention, the related metabolic changes in the occurrence and development process of the old-coagulation blood-stasis symptoms and the treatment process of the Chinese angelica-based cold-coagulation blood-stasis treatment decoction are studied by using a dynamic analysis method; the disease occurrence and development mechanisms at different time points can be revealed;and the abnormal metabolic network regulated and controlled by medicines in the treatment process can be clarified.
Owner:GUANGXI MEDICAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com