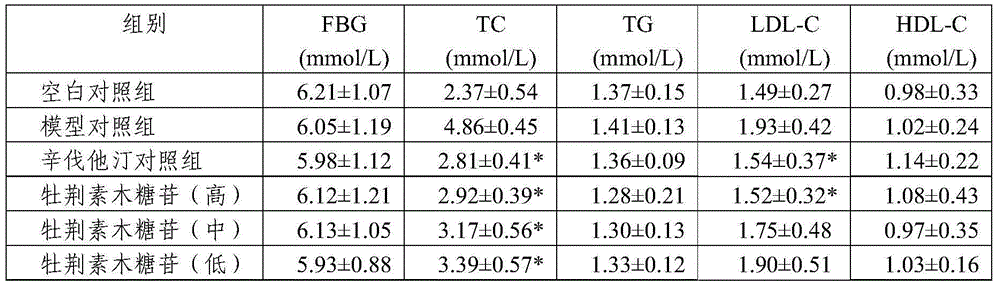
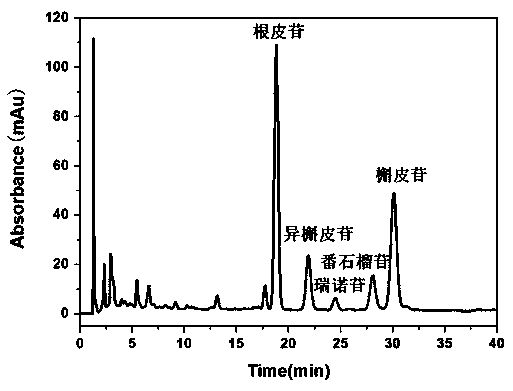
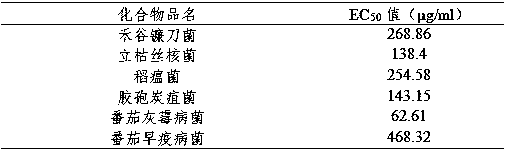
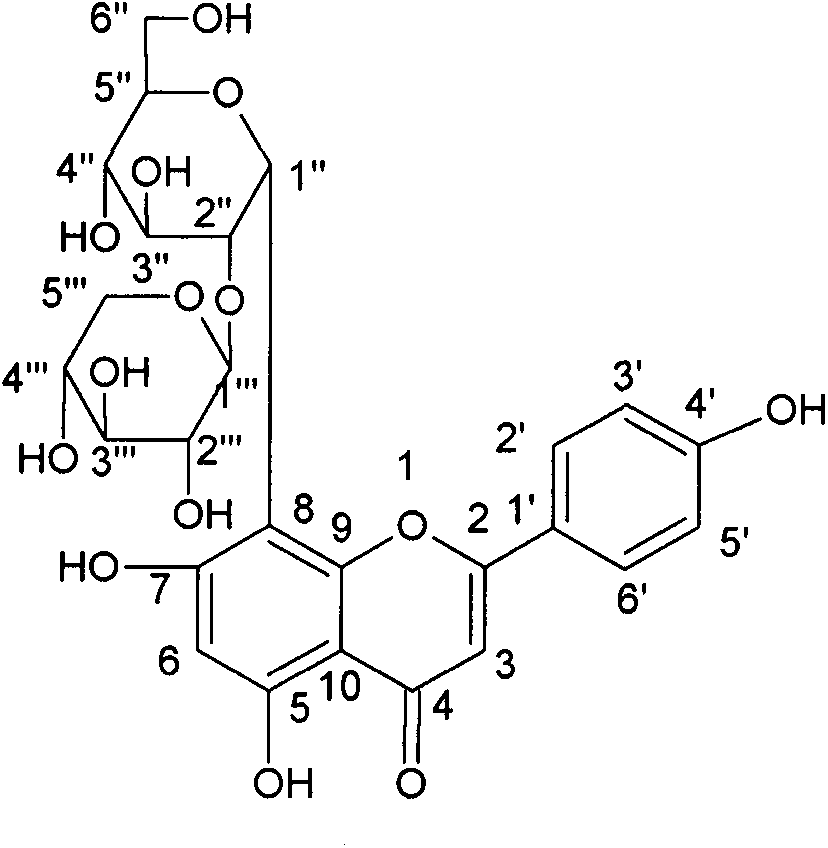
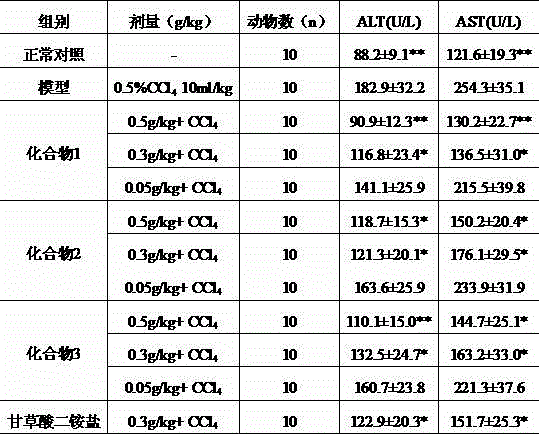
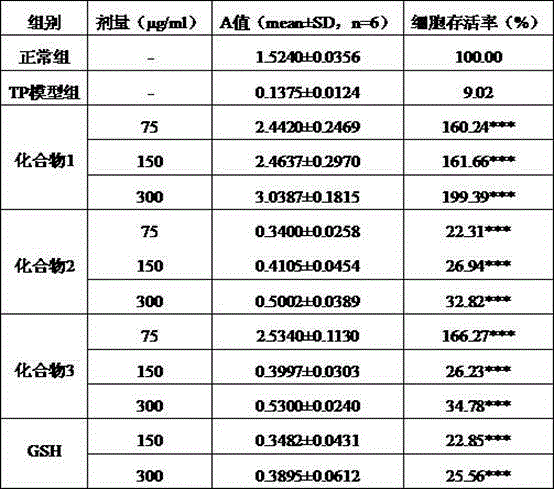
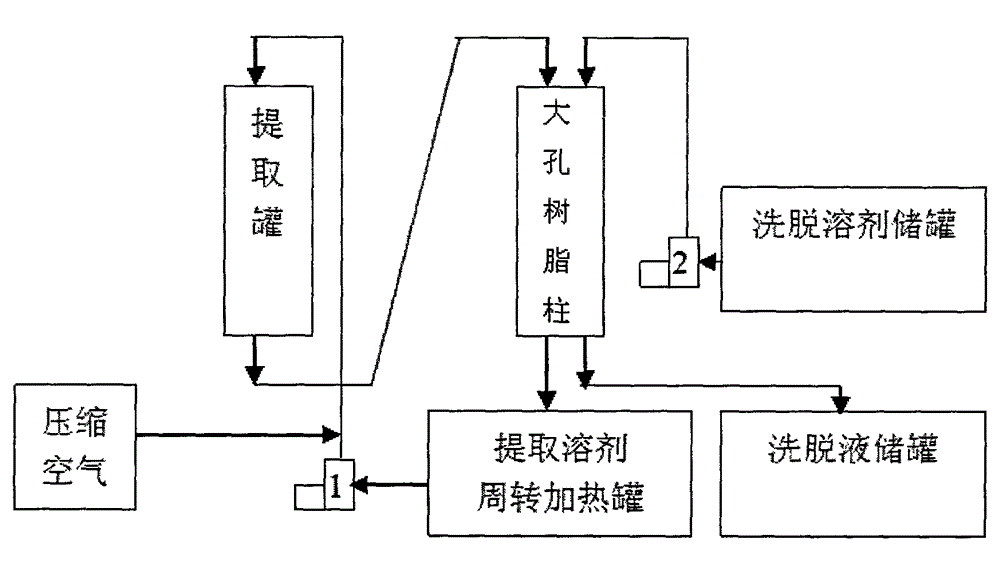
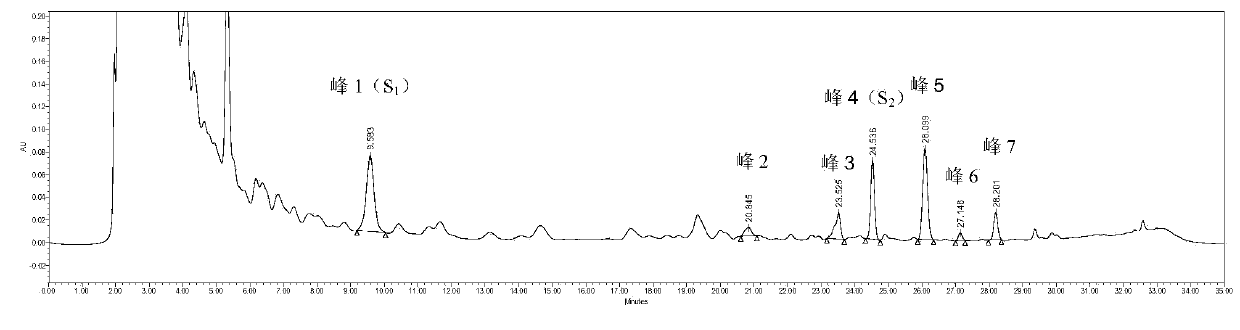
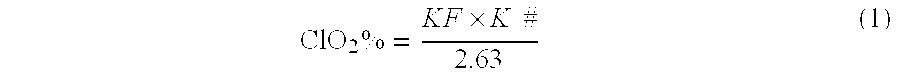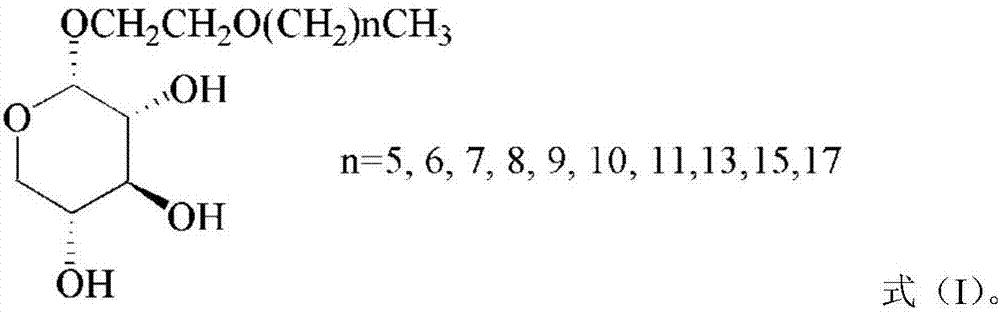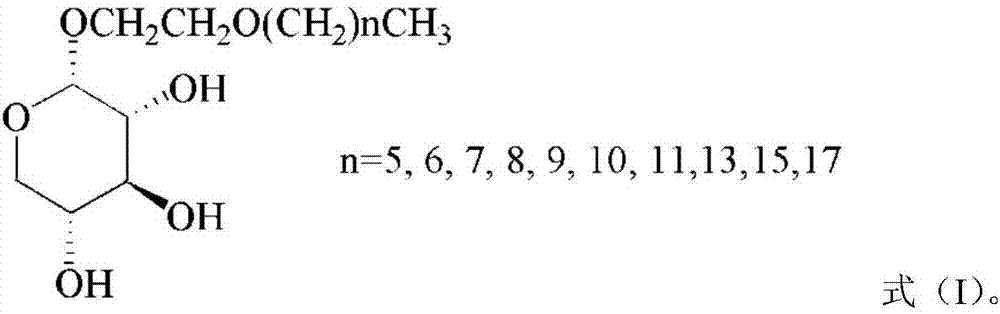Patents
Literature
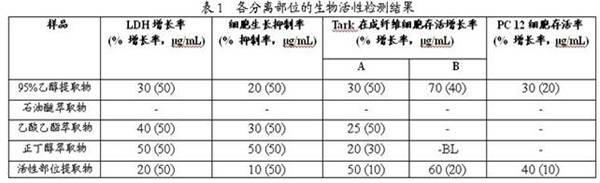
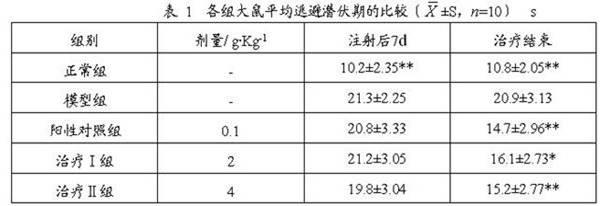
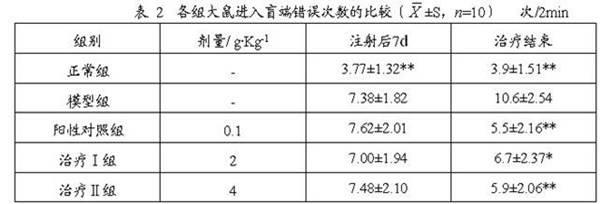
63 results about "Xyloside" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A xyloside is a type of glycoside derived from the sugar xylose. Proteoglycan (PG) synthesis is initiated by the transfer of D-xylose from UDP-xylose to a serine residue in core proteins. This natural primer acts as a template for the assembly of heparin sulfate, heparin, chondroitin sulfate, and dermatan sulfate side chains, depending on the tissue. However, in 1973 it was determined that synthetic B-D-xylosides can prime glycosaminoglycan (GAG) synthesis by substituting for the core xylosylated protein.
Xylanases, Nucleic Acids Encoding Them and Methods For Making and Using Them
ActiveUS20110016545A1Low cost processingLow-cost and efficientImmobilised enzymesNon-fibrous pulp additionIncrease phCell wall
The invention relates to enzymes having xylanase, mannanase and / or glucanase activity, e.g., catalyzing hydrolysis of internal β-1,4-xylosidic linkages or endo-β-1,4-glucanase linkages; and / or degrading a linear polysaccharide beta-1,4-xylan into xylose. Thus, the invention provides methods and processes for breaking down hemicellulose, which is a major component of the cell wall of plants, including methods and processes for hydrolyzing hemicelluloses in any plant or wood or wood product, wood waste, paper pulp, paper product or paper waste or byproduct. In addition, methods of designing new xylanases, mannanases and / or glucanases and methods of use thereof are also provided. The xylanases, mannanases and / or glucanases have increased activity and stability at increased pH and temperature.
Owner:BP CORP NORTH AMERICA INC
Fibrized cellulomonas cartae, hydrolase and application thereof in taxane conversion aspect
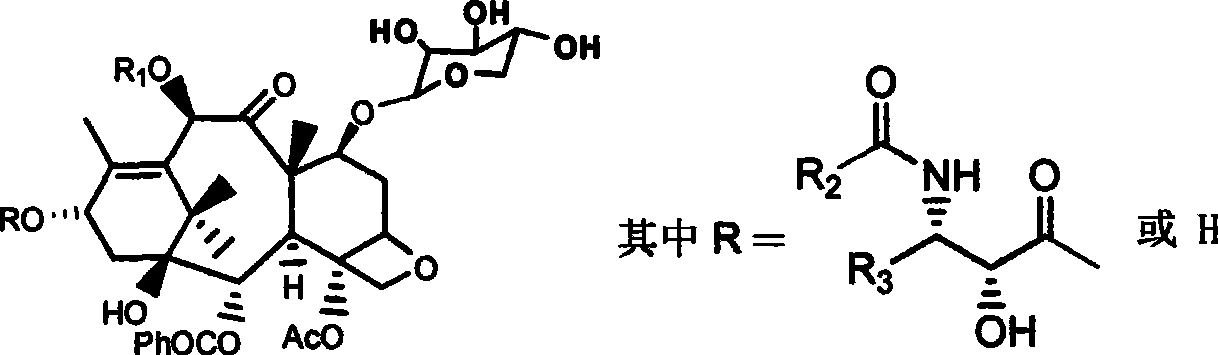
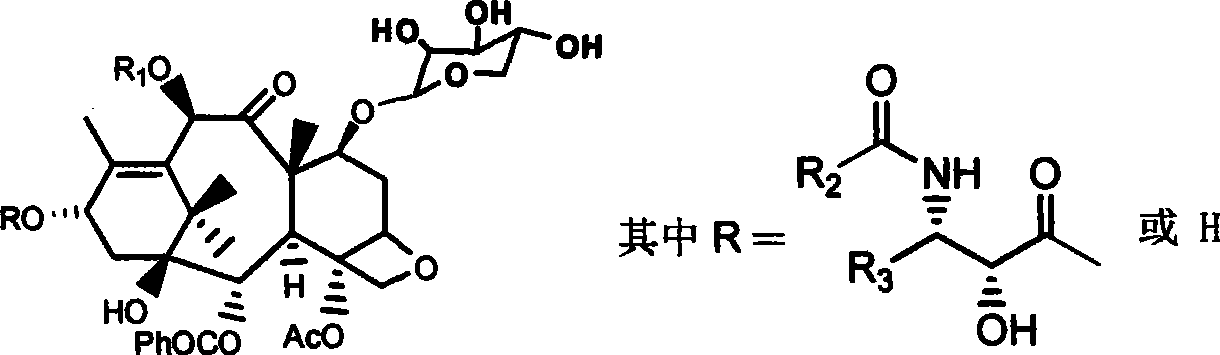
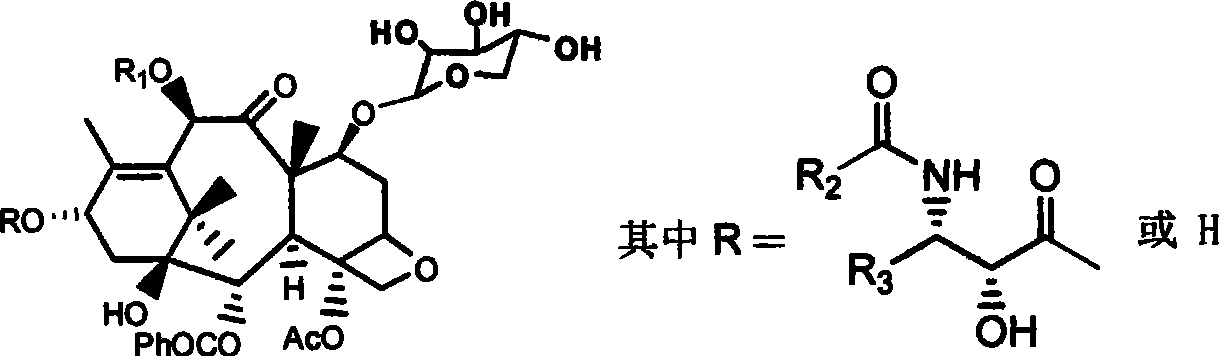
The invention provides a fibration Cellulomonas spp and a hydrolytic enzyme thereof for effectively transforming taxane xyloside into taxol or the analogues thereof, and an effective new method for preparing the taxol or the analogues thereof through biotransformation reaction. The method can prepare the taxol or the analogues thereof with high efficiency, low cost and environmental protection, which provides an effective approach adapting to industrial production for making full use of taxane resources.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
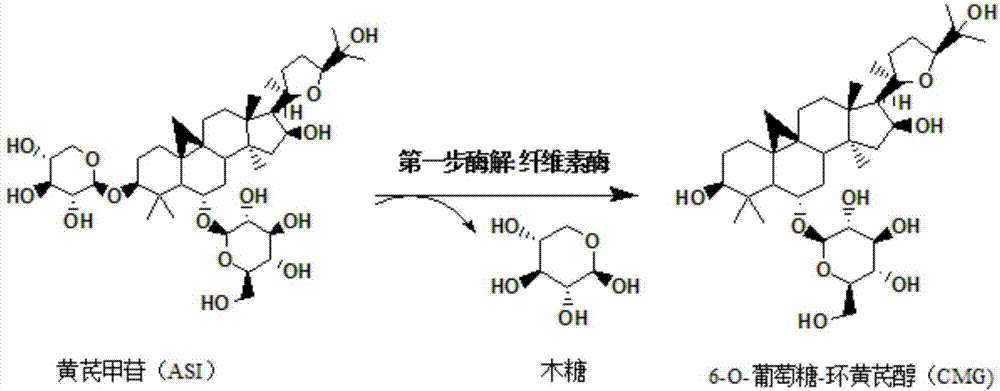
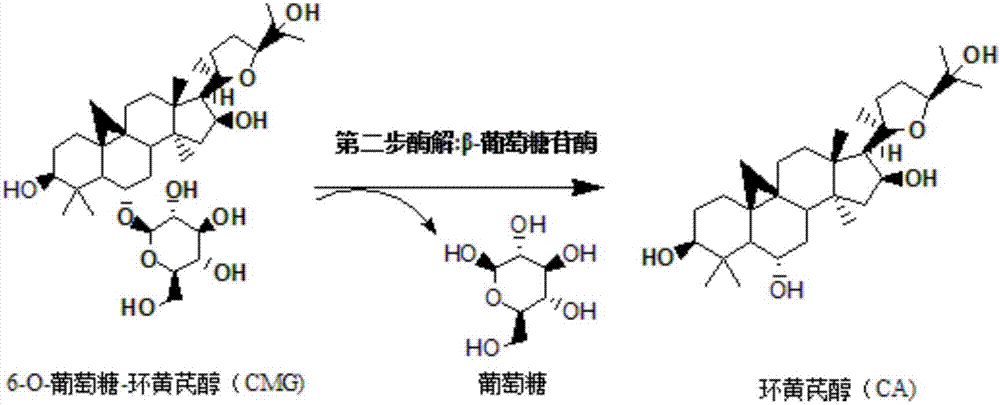
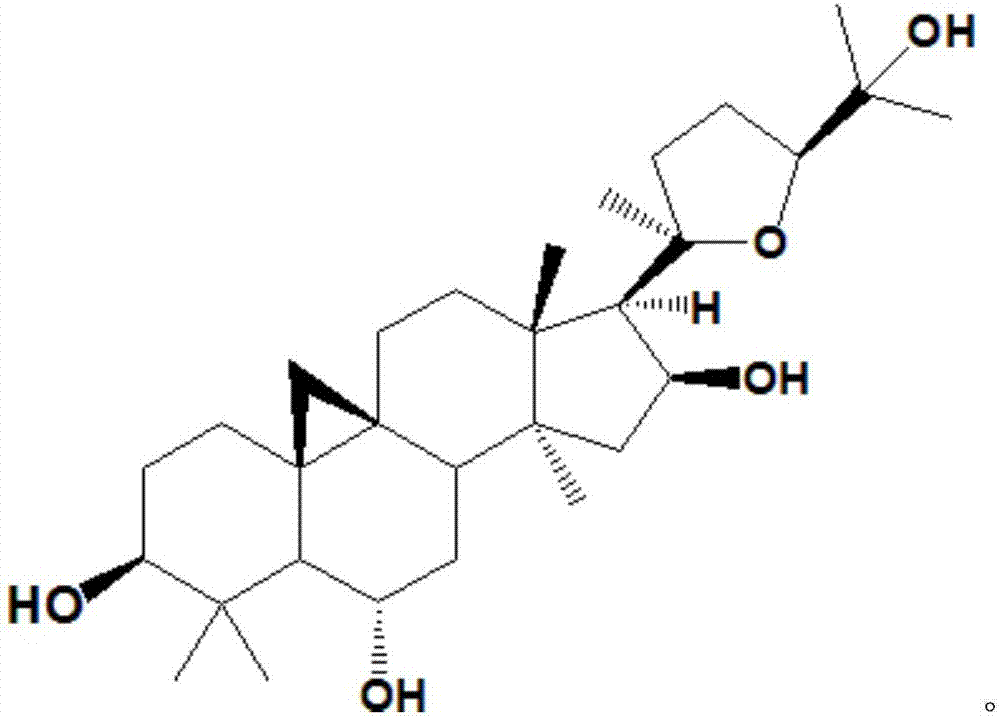
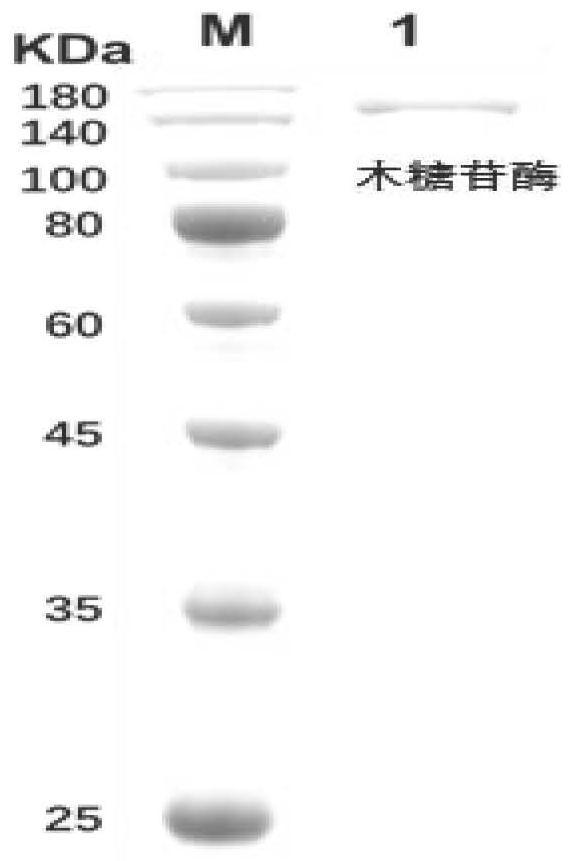
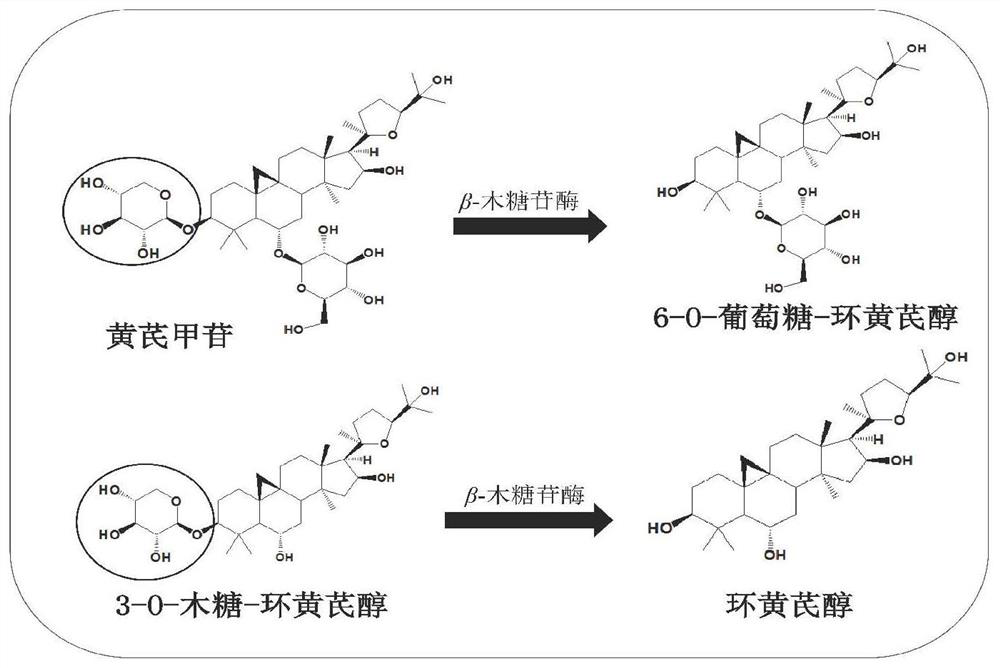
Method for converting astragalosides to prepare cycloastragenol by two-step enzymolysis
The invention discloses a method for converting astragalosides to prepare cycloastragenol by two-step enzymolysis; the method comprises the specific steps of using astragalosides as a substrate, using two different hydrolases to hydrolyze and break beta-xyloside bond at site C3 of the astragalosides and beta-glucoside bond at the site C6 respectively to obtain aglycone cycloastragenol of the astragalosides, extracting, performing silica-gel column chromatography, recrystallizing with ethanol, and purifying to obtain the finished cycloastragenol up to 95% and higher in purity. The problem that damage of three-membered ring structure of astragalosides during the preparation of cycloastragenol by a chemical process causes massive byproducts is solved, the defects of traditional cycloastragenol preparation methods, such as low substrate conversion rate, step complexity and environmental pollution, are overcome. The method has the advantages that substrate specificity is high, the substrate astragalosides is completely converted, the steps are simple, the conditions are mild, the cost is low, and the method is a mild biological preparation method, has no environmental pollution and is suitable for industrial production.
Owner:BEIJING UNIV OF CHEM TECH
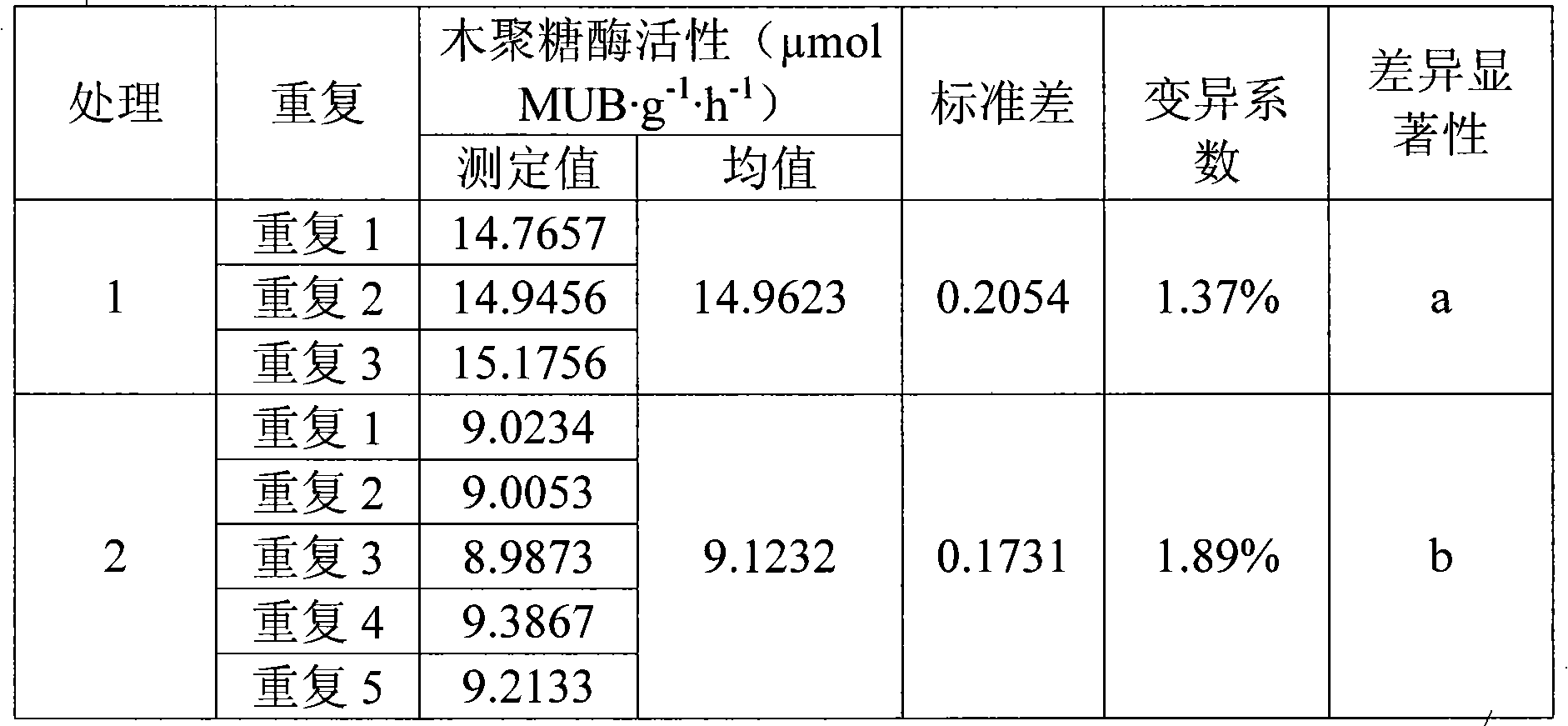
Analyzing method for detecting activity of soil xylanase
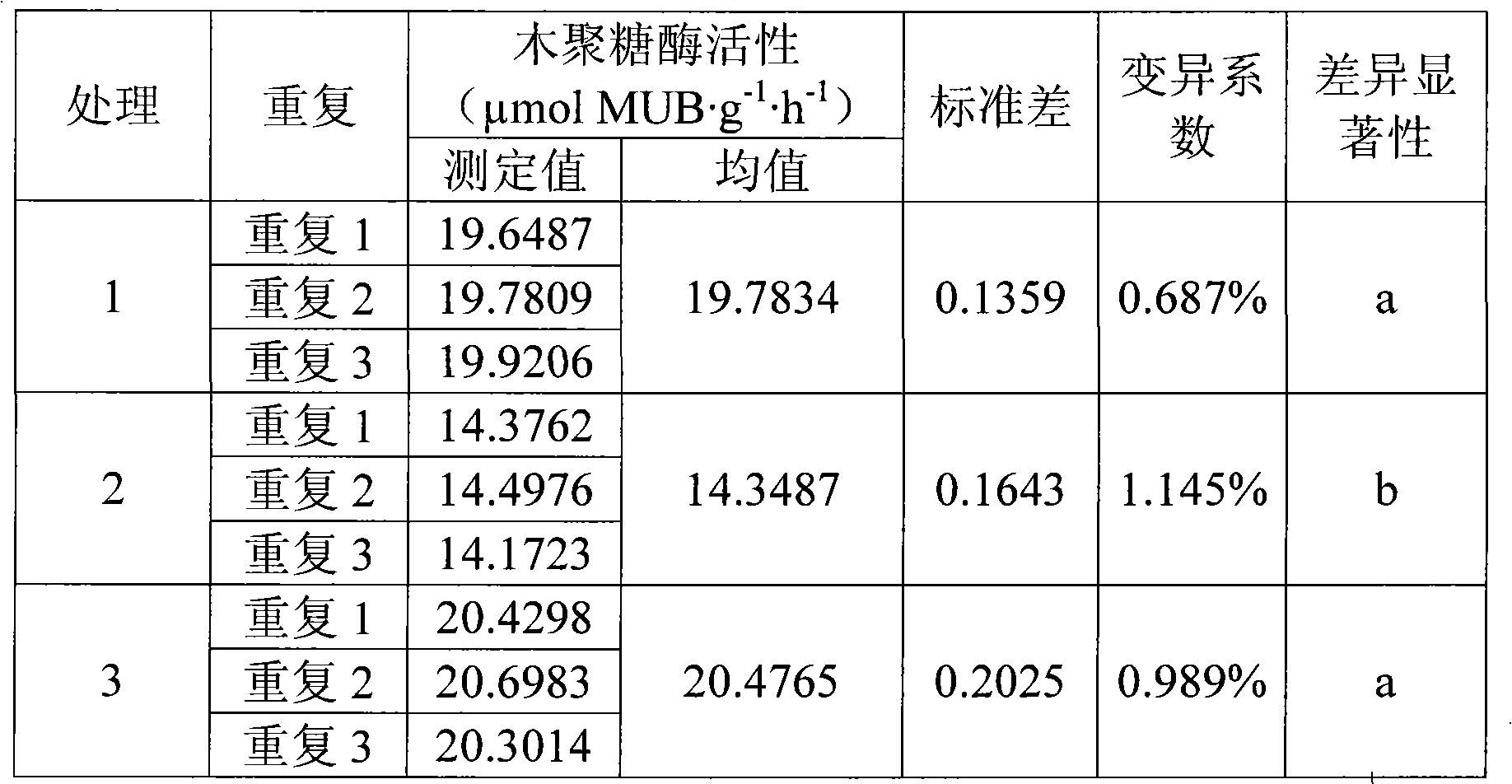
InactiveCN101586145AReduce incubation timeShort training timeMicrobiological testing/measurementFluorescence/phosphorescenceFiltrationExoxylanase activity
The invention relates to an analyzing method for detecting activity of soil xylanase, which comprises the following steps: firstly, weighting n sieved air dry soil samples into n thick test tubes, adding acetic acid buffer solution into each test tube, oscillating by a vortex oscillator, getting soil suspension into 96 micropore plates under the oscillation condition, adding 4-MUB-7-Beta-D-xyloside substrate solution into n-1 holes, and adding water with equal quantity into the other one hole so as to be used as non-substrate contrast, adding substrate solution with equal quantity and water with equal quantity into the (n+1)th hole so as to be used as soli-free contrast, oscillating and culturing under constant temperature; secondly, adding NaOH into the micropore plates to terminate the reaction after the culture is finished; thirdly, performing the fluorimetric determination to resultant of reaction by a multifunctional microplate reader; and fourthly, calculating the activity of the xylanase. Compared with the traditional method, the invention shortens the culture time, omits the operation procedures of filtration, and the like, and simplifies the operation steps; meanwhile, the determination data of fluorescent materials in the micropore plate can be obtained within 15s through the multifunctional microplate reader, huge samples are allowed to be simultaneously determined; and moreover, the invention has high accuracy and easy operation, stable and reliable result and good reproduction quality.
Owner:SHENYANG INST OF APPL ECOLOGY CHINESE ACAD OF SCI
Anti-inflammatory cranberry flavonol extract preparations
The present invention is directed to extracts of cranberries (Vaccinium macrocarpon) comprising either mixed flavonols that are substantially free of anthocyanins and proanthocyanidins or a purified cranberry flavonol compound, including myricetin-3-β-xylopyranoside, quercetin-3-β-glucoside, quercetin-3-α-arabinopyranoside, 3′-methoxyquercetin-3-α-xylopyranoside, quercetin-3-O-(6″-p-coumaroyl)-β-galactoside, and quercetin-3-O-(6″-benzoyl)-β-galactoside. The present invention also embodies the use of those extracts, as well as extracts comprising the cranberry flavonol compound quercetin-3-α-arabinofuranoside, for the treatment of inflammatory disorders. Pharmaceutical, food, dietary supplement, and cosmetic compositions utilizing the extracts or compounds of the present invention are also recited.
Owner:VORSA NICHOLI +4
Method for measuring content of ellagic acid ingredients in euscaphis japonica medicinal materials
ActiveCN101788537AQuality improvementHigh precisionComponent separationPlant ingredientsMedicinal herbsAdditive ingredient
The invention provides a method for measuring content of ellagic acid ingredients in euscaphis japonica medicinal materials, which synchronously measures the contents of at least two of five ingredients of ellagic acid, 3,3'-dimethoxy ellagic acid, 3,3'-dimethoxy ellagic acid-4'-O-beta-D-xyloside, 3,3'-dimethoxy ellagic acid-4'-O-beta-D-glucoside and 3,3'-dimethoxy ellagic acid-4'-O-alpha-D-arabinoside contained in the euscaphis japonica medicinal materials by using a high efficiency liquid chromatography or an ultra-high efficiency liquid chromatography under the same chromatogram condition. In the invention, the contents of the multiple ingredients in the euscaphis japonica medicinal materials are measured under the same chromatogram condition so that the method for measuring the content of the ellagic acid ingredient in the euscaphis japonica medicinal materials has better integrity, characteristics and stability as well as high precision, favorable repeatability and high recovery rate and capability of effectively controlling the quality of the euscaphis japonica medicinal materials, thereby ensuring the effectiveness and the safety of clinical medication and laying the foundation for redevelopment and application of the euscaphis japonica medicinal materials.
Owner:贵州益康制药有限公司
Novel 1,2-cis-xyloside surfactant
InactiveCN106883276ANovel structureRaw materials are easy to getSugar derivativesTransportation and packagingSolubilityGlycoside formation
The invention discloses a novel xylose-derived 1,2-cis glycoside surfactant and application thereof. 1,2-cis alkoxy ethyl-alpha-D-pyranxyloside is obtained from D-xylose through three steps of reactions, i.e., acylation, coupling and deprotection. According to the surfactant, D-xylose obtained from crop leftovers can be thoroughly converted, the production cost is low, the preparation method is simple, and the product is environmentally friendly. The glycoside is improved in water solubility, relatively high in surface activity and optional and controllable in foamability and emulsifying property and has good market prospect and economic value as a surfactant.
Owner:XIANGTAN UNIV
Application of flavone glycoside compounds in preparing medicament for treating and preventing hepatitis
InactiveCN102379888AImprove in vitro damageSignificant improvementOrganic active ingredientsDigestive systemApigeninGlucoside
The invention belongs to the field of pharmacy, and provides application of three flavone glycoside compounds luteolin-7-O-[alpha-L-rhamnose-(1->2)]-beta-D-glucoside (trivial name is lonicerin), apigenin-7-O-[alpha-L-rhamnose-(1->2)]-beta-D-glucoside (trivial name is rhoifolin) and luteolin-6-C-beta-D-glucose-8-C-beta-D-xyloside in preparing medicament or health-care food for preventing and treating hepatitis.
Owner:JIANGXI UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
UPLC method for detecting components in radix puerariae, radix puerariae extract and radix puerariae-containing preparation
ActiveCN106370763AAccurate quality controlShorten the timeComponent separationMedicinal herbsPuerarin
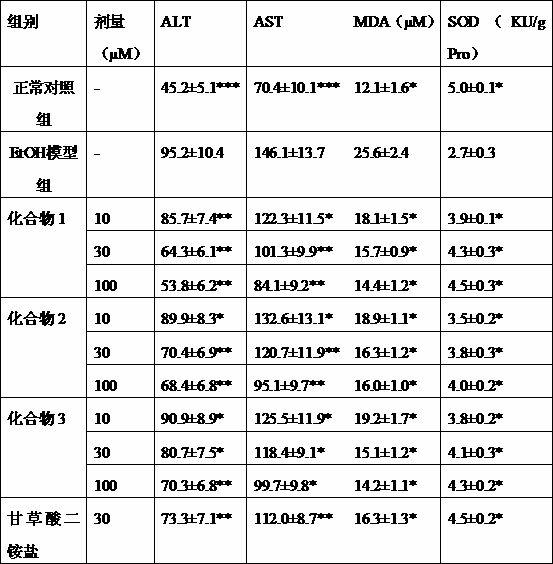
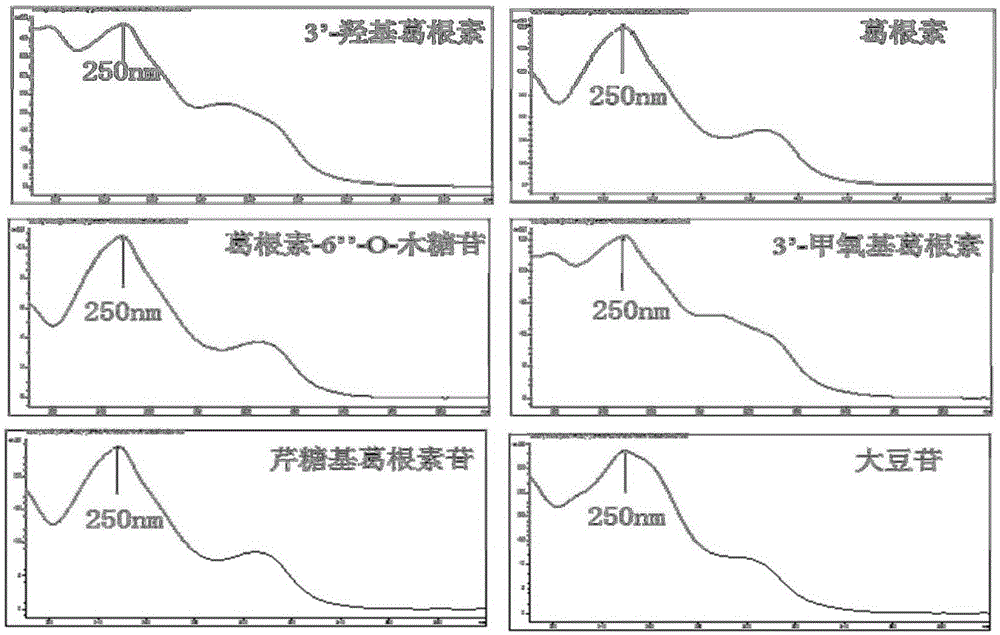
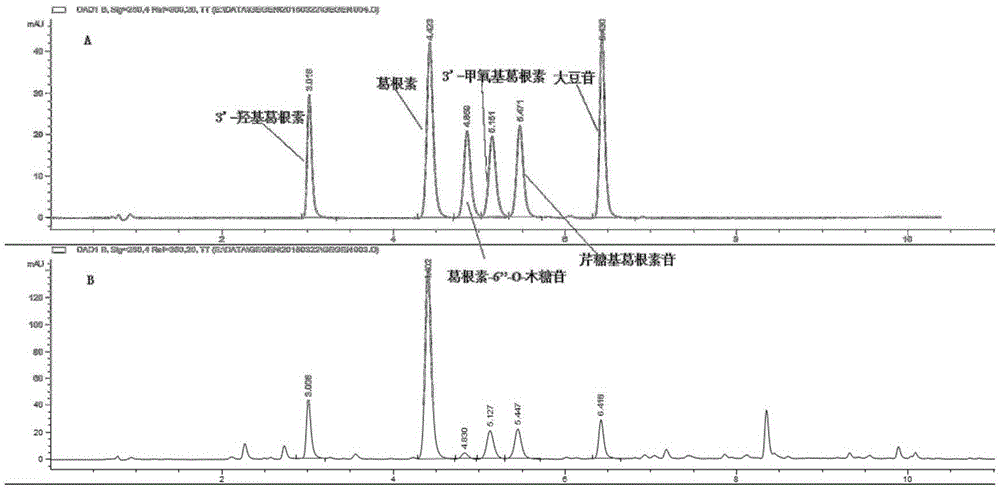
The present invention provides a UPLC method for qualitatively and quantitatively detecting the contents of six components such as 3'-hydroxy puerarin, puerarin, puerarin-6"-O-xyloside, 3'-methoxy puerarin, mirificin and daidzin in radix puerariae, radix puerariae extracts and radix puerariae-containing preparations by adopting puerarin as a reference substance. The UPLC method comprises: preparing a reference substance solution and a testing sample solution, and carrying out gradient elution by adopting 0.1% acetic acid as a mobile phase A and adopting acetonitrile as a mobile phase B, wherein the detection wavelength is 250 nm, the chromatographic column is SB-C18, RRHD 1.8 [mu]m, 2.1*100 mm, the column temperature is 31 DEG C, the flow rate is 0.4 mL / min, and the injection volume is 1 [mu]L; comparing the obtained chromatogram with a radix puerariae herb finger print, wherein the main peak is puerarin, and other components are corresponding to various peaks in a one-to-one manner so as to identify the herb; and calculating the contents of the six components in the radix puerariae by combining relative calibration factors (the relative calibration factor of 3'-hydroxy puerarin is 1.253, the relative calibration factor of puerarin is 1.000, the relative calibration factor of puerarin-6"-O-xyloside is 1.422, the relative calibration factor of 3'-methoxy puerarin is 1.297, the relative calibration factor of mirificin is 1.332, and the relative calibration factor of daidzin is 1.030) and the peak area of puerarin in the contrast solution.
Owner:HUAZHONG UNIV OF SCI & TECH
Preparation method for obtaining uniform xylan from corn cob
The invention relates to the field of the preparation of xylan, and particularly relates to a method for preparing and purifying uniform xylan from a corn cob. A framework is formed by xylose repeating units through the connection of beta-D-1,4 xyloside bonds, and xylose residues account for 99% or above. In addition to xylose, the xylan prepared in the prior art also has other monosaccharide accounting for a certain percentage, such as galactose, glucose, arabinose, glucose and some uronic acids. According to the method, 99% (or above) of monosaccharide components of the xylan prepared through a special process are xylose. The method comprises the following specific steps: crushing the corn cob; sieving with a 20-mesh sieve; and performing alkaline extraction at high temperature, wherein the solid-to-liquid ratio is (1:2)-(1:20), the concentration of an alkaline solution is 2-20%, and the reaction time is 1-6 hours. According to the invention, the main chain of the prepared xylan is composed of 2-3 hundreds of xylose residues; and through the connection of beta-1,4 glycosidic bonds, the problem that other miscellaneous monosaccharide is contained when xylo-oligosaccharide is obtained through the degradation of xylan is well solved. Thus, the invention has good application prospects.
Owner:CHINA PHARM UNIV
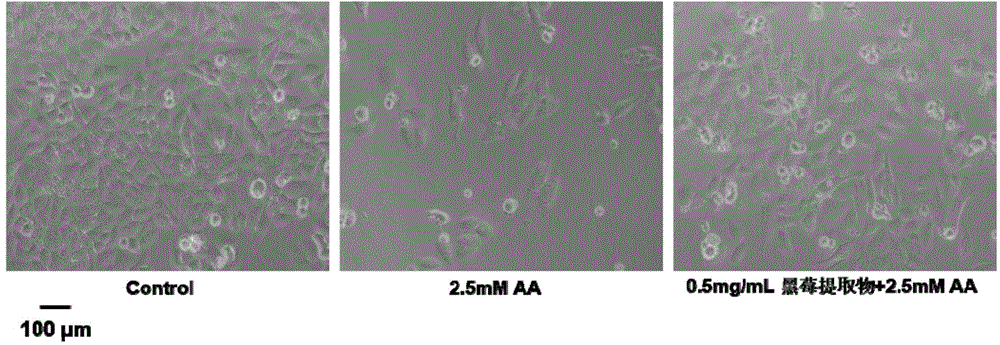
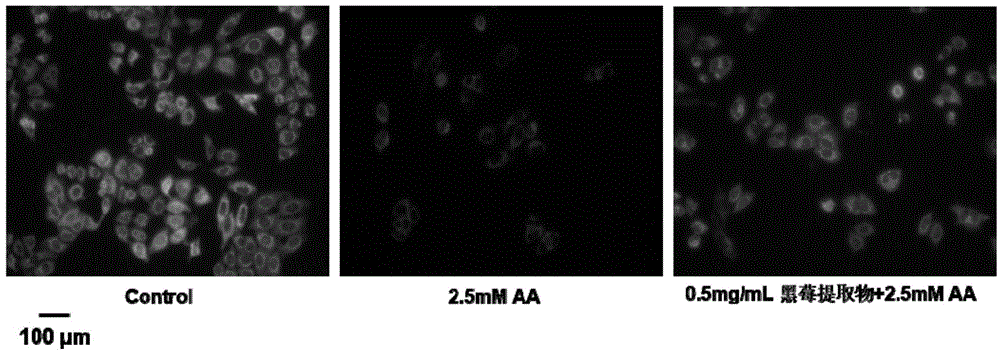
Blackberry extract and application of blackberry extract in preparation of liver cell oxidative damage inhibitor
ActiveCN104606306AReduce generationOrganic active ingredientsDigestive systemAdditive ingredientPepsin
The invention discloses a blackberry extract. The blackberry extract comprises components in percentage by mass as follows: 58.3%-72.5% of cyanidin-3-glucoside, 11.6%-15.8% of cyanidin-3-xyloside, 9.8%-14.6% of cyanidin-3-oxalyl glucoside, 3.1%-6.4% of cyanidin-3-malonyl glucoside, 2.7%-4.1% of delphinidin-3-xylose and 0.3%-0.8% of cyanidin-3-arabinfuranoside. The extraction steps are as follows: after enzymolysis of blackberry fresh fruits by pepsase and trypsin sequentially, centrifugal separation is performed, a liquid supernatant is obtained and filtered by an ultrafiltration membrane, ingredients with molecular weight larger than 10 kDa are cut off, filtrate is subjected to vacuum freezing drying, and the blackberry extract is obtained. The invention further discloses an application of the blackberry extract in preparation of a liver cell oxidative damage inhibitor.
Owner:ZHEJIANG UNIV
Extract of active anti-fatigue part of okra, and preparation method and application thereof
The invention discloses an extract of an active anti-fatigue part of okra, and a preparation method and application thereof. The extract of the active anti-fatigue part of okra uses total polyphenols as a main active anti-fatigue component, wherein the total polyphenols account for 11.79 to 23.34% of the total mass of the extract of active anti-fatigue part and mainly comprise catechin derivatives and quercetin derivatives, and the quercetin derivatives are mainly composed of quercetin-3-O-gentiobioside, quercetin-3-O-glucose-xyloside, isoquercitrin, quercetin-3-O-malonyl-glucoside, rutin and quercetin. The extract of the active anti-fatigue part of okra provided by the invention can substantially prolong the load swimming time of mice, eliminate lactic acid accumulation after sports, reduce the content of serum urea nitrogen, increase hepatic glycogen and improve the antioxidation capability of mice in a stress state, and has substantial anti-fatigue effect.
Owner:INST OF MEDICINAL PLANT DEV CHINESE ACADEMY OF MEDICAL SCI
Method for separating three tannin monomer components from garden burnet
ActiveCN103913522AImprove separation efficiencyFast separationComponent separationGallic acid esterTannin
The invention provides a method for separating three tannin monomer components from garden burnet. The three tannin monomer components comprises gallic acid, 3,3',4'-O-trimethylellagic acid-4-O-beta-D-xyloside and 3,3',4'-O-trimethylellagic acid. The method utilizes a preparative high performance liquid chromatography to separate and prepare the tannin monomer components, realizes purity more than 98%, has high separation efficiency and a fast separation rate and has a good industrial prospect. The tannin monomer components can be used as tannin standards and can be widely used in research fields of drugs, pharmaceutical analysis and pharmacology.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
Antineoplastic effect of a group of cycloart-one triterpene compound
InactiveCN1524532AStrong killing effectGood at scavenging free radicalsOrganic active ingredientsPharmaceutical delivery mechanismTriterpeneTriterpenoid
The invention relates to a the antineoplastic action of a group of looped pineapple confectionery terpenoid, which is three 9, 19 looped lanolinum alkyl triterpenoid (23-oxygen-acetyl cimicifugol - 3 -oxygen - beta - D - xyloside, 24 - oxygen - acetyl cimicifugol - 3 -oxygen-beta-D-xyloside and 25-oxygen-acetyl cimicifugol - 3 -oxygen - beta - D - xyloside) extracted from cimicifuga rhizome. Experimental investigation has shown that they have cell toxic action for blood tumor and entity tumor.
Owner:肖培根 +1
Viili extracellular polysaccharide and preparation method thereof
The invention relates to a viili extracellular polysaccharide and a preparation method thereof. The extraction of the viili extracellular polysaccharide mainly comprises: enzymolysis, deproteinization, alcohol precipitation and other steps. The separation and purification of extracellular polysaccharide mainly comprises ion-exchange column chromatography and gel filtration chromatography. The invention also discloses the composition of the viili extracellular polysaccharide, which is that the viili extracellular polysaccharide mainly comprises rhamnose, L-arabinose, xylose, mannose, glucose and galactose in a molar ratio of 7.41:15:8.85:1:3.25:1.25. The monosaccharide residue of the viili extracellular polysaccharide exists in form of pyranoid ring and furan ring. The preparation method and product of the viili extracellular polysaccharide are suitable to be used as liposome nanometer materials and functional factors for functional foods and helps to develop some special medicine carrier materials and novel functional foods.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
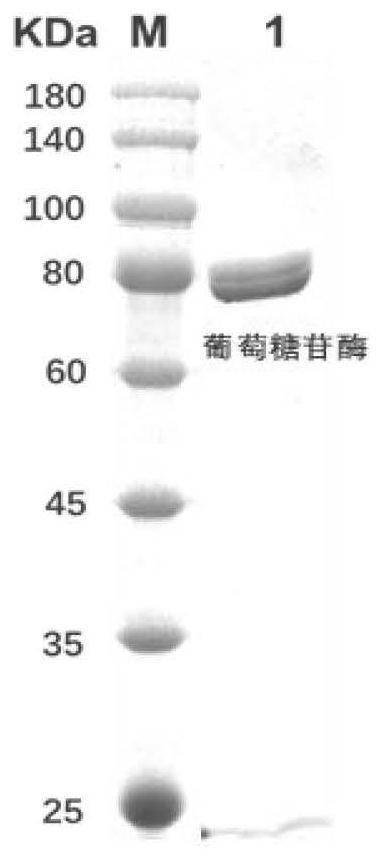
Method for preparing cycloastragenol by double-enzyme compounding conversion of astragaloside
ActiveCN111893158AIncreased substrate toleranceHigh substrate conversion rateMicroorganism based processesFermentationAstragalosideAlglucerase
The invention relates to the technical field of biotransformation, in particular to a method for preparing cycloastragenol through double-enzyme compounding transformation of astragaloside. Accordingto the method, the astragaloside is used as a substrate, xylosidase and glucosidase are subjected to double-enzyme compounding, then xyloside bonds at the C3 position of the substrate and glucoside bonds at the C6 position of the substrate are broken through one-step hydrolysis, and the cycloastragenol is obtained. The purity of the obtained cycloastragenol can reach 98% or above, and the method is easy to operate, free of pollution, milder in reaction temperature, clear in enzyme conversion mechanism, and wider in enzyme substrate adaptability and is suitable for industrial production.
Owner:WEIHAI BAIHE BIOTECH +1
Cellulose degrading enzyme with glucosidase/xylosidase dual functions and preparation method and application thereof
InactiveCN101870966APromote digestionHigh purityBiofuelsAnimal feeding stuffAdditive ingredientBio engineering
Owner:FUDAN UNIV
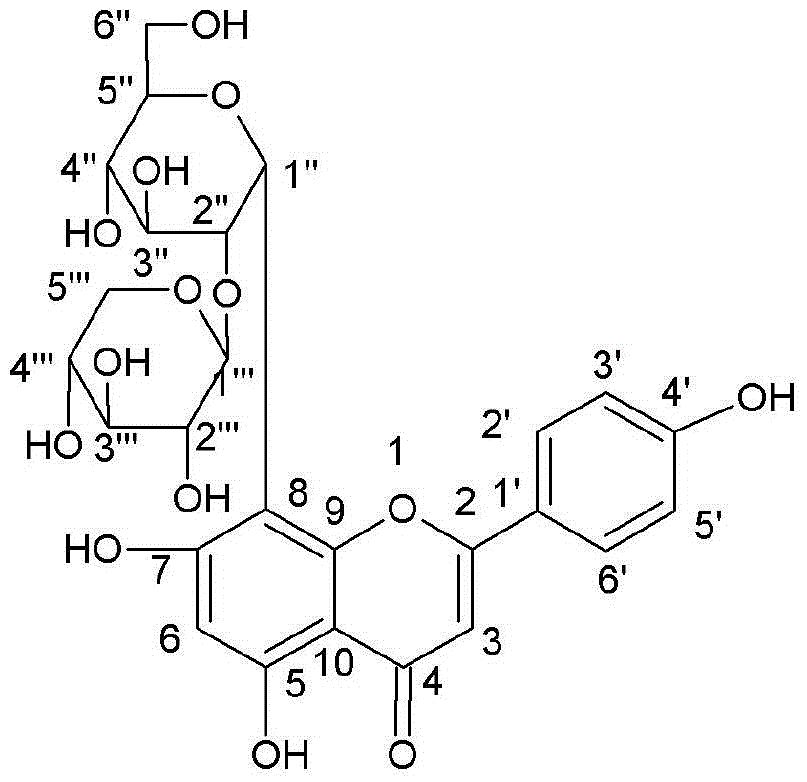
New use of vitexin xyloside
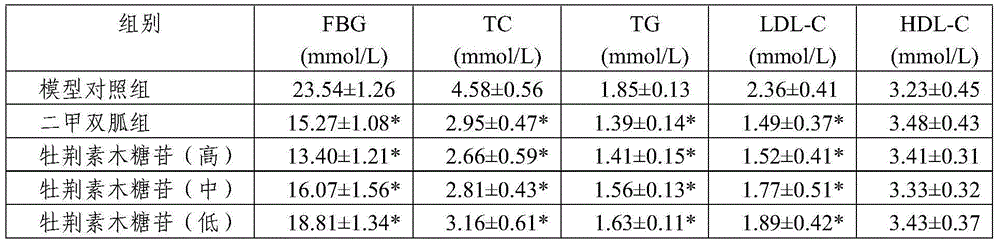
ActiveCN104958311AReduce complicationsReduce morbidityOrganic active ingredientsMetabolism disorderAcute hyperglycaemiaSecondary hyperlipidemia
The invention relates to a new use of vitexin xyloside, and concretely relates to an application of the vitexin xyloside in the preparation of medicines for treating metabolic diseases. The metabolic diseases are hyperlipidemia or hyperglycemia, and complications thereof. A test result shows that the vitexin xyloside has obvious diabetic mouse blood sugar and blood fat reducing effects, and is better than substances in the prior art, and the action of the vitexin xyloside has a certain dose-effect relationship.
Owner:GUANGXI UNIV OF CHINESE MEDICINE
Application of apple residue and apple leaf extract to prevention and control of phytopathogen
InactiveCN109042651AStrong antibacterial activityGood control effectBiocideFungicidesSingle substanceQuercitrin
The invention relates to application of an apple residue and apple leaf extract to prevention and control of phytopathogen. The apple residue and apple leaf extract contains polyphenols of phlorizin,quercitrin, isoquercitrin, quercetin-3-D-xyloside and quercetin-3-D-glucosidase arabinofuranoside, and can be applied to prevention and control of diseases caused by fungi and diseases caused by bacteria for various grain crops, economic crops, vegetables, fruit trees, melons and the like. A biological sterilizing preparation prepared by extracting a mixture or a single substance is environmentally friendly, is safe to human beings and livestock, and can be used for effectively preventing and controlling various plant diseases.
Owner:BEIJING UNIV OF CHEM TECH
Method for extracting and separating vitexin xyloside from natural product
ActiveCN104974202AImprove extraction efficiencyHigh puritySugar derivativesSugar derivatives preparationNatural productVitexin
The invention relates to a method which is easy for industrial separation and purification of vitexin xyloside. The method has high extraction efficiency. The technology comprises steps as follows: extraction of ethanol, macroporous resin column chromatography and preparation of high performance liquid chromatography for separation and purification. Purity of vitexin xyloside prepared by the method reaches more than 90%. The preparation method is safe, simple and low-cost, and is suitable for industrial production.
Owner:GUANGXI UNIV OF CHINESE MEDICINE
Discocleidion sufescens Pax et Hoffm. extract, preparation method thereof and purpose thereof
ActiveCN102485231AOrganic active ingredientsNervous disorderTraditional medicinePharmaceutical Substances
The invention relates to a Discocleidion sufescens Pax et Hoffm. extract, a preparation method thereof and a purpose thereof. The extract comprises the following six ellagic acid compounds: ellagic acid (I), 3,3',4'-trimethyl ellagic acid (II), 3,3'-dimethyl ellagic acid (III), 3,3',4-trimethyl ellagic acid-4'-O-beta-D-glucoside (IV), 3,3'-dimethyl ellagic acid-4'-O-beta-D-glucoside (V), and 3,3'-dimethyl ellagic acid-4'-O-beta-D-xyloside (VI). The extract can be applied to prepare senile dementia resisting medicines.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
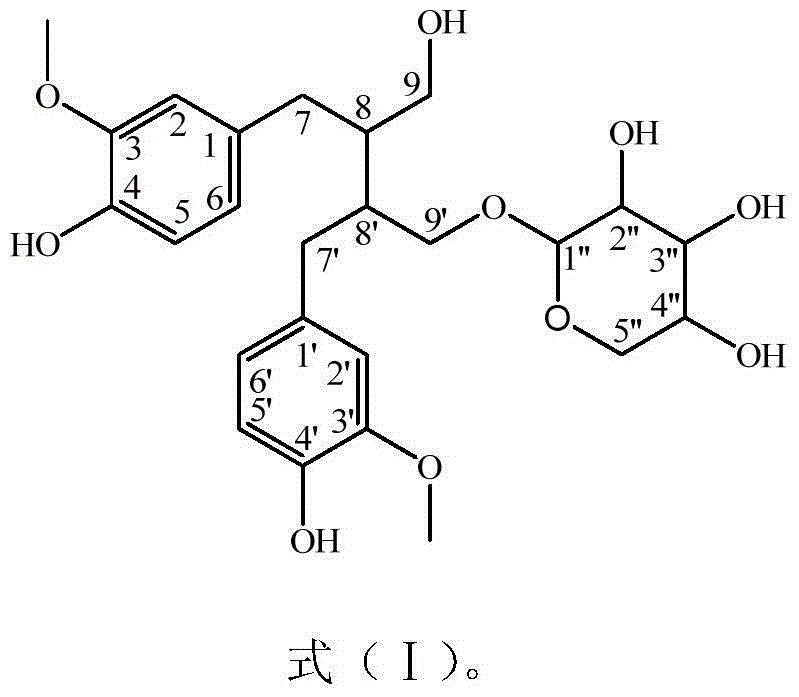
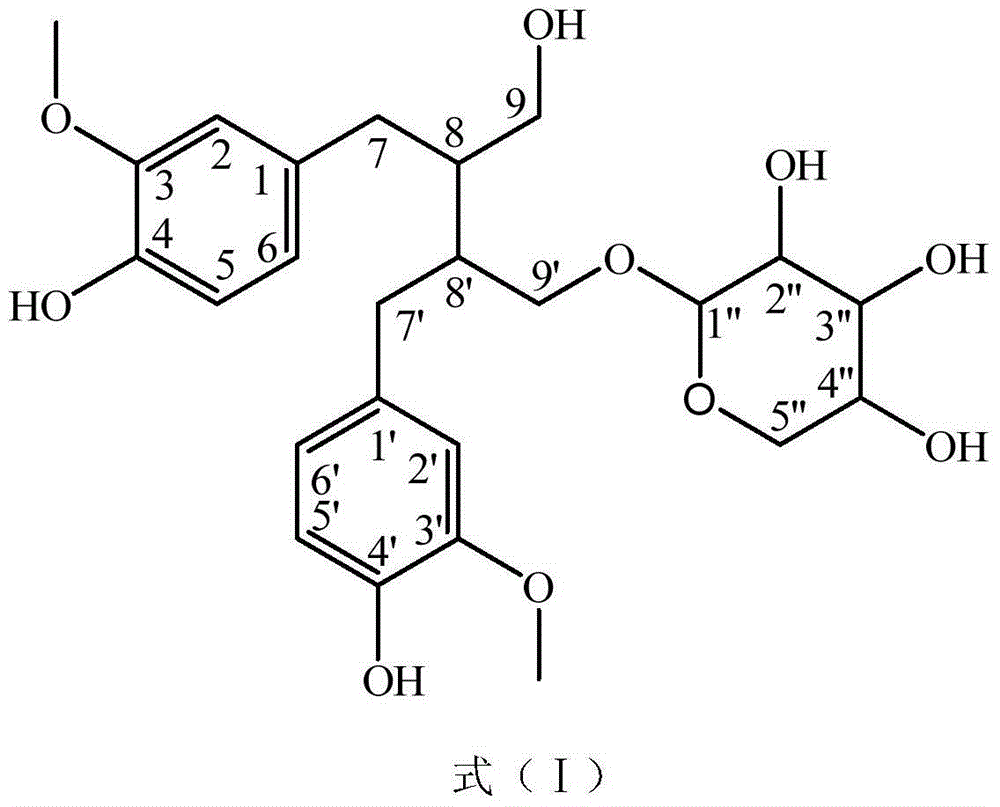
Method for preparing secoisolariciresinol 9'-O-beta-xyloside
ActiveCN104004034AIncrease added valuePromote sustainable developmentSugar derivativesSugar derivatives preparationSecoisolariciresinolStructural formula
The invention discloses a method for preparing secoisolariciresinol 9'-O-beta-xyloside. The structural formula of secoisolariciresinol 9'-O-beta-xyloside is as shown in formula (I). According to the invention, a lot of secoisolariciresinol 9'-O-beta-xyloside is prepared and separated from lichee leaves, and the yield is 20-85 mg / kg (the purity is 85-95%). The method of the invention has important significance on promoting lichee deep processing and utilization, increasing the added value lichee products, and promoting the sustainable development of the industry.
Owner:SOUTH CHINA BOTANICAL GARDEN CHINESE ACADEMY OF SCI
Use of alkylpolyxylosides in cosmetics
InactiveUS7652130B2Enhances cosmetic feelCosmetic preparationsOrganic active ingredientsPhotochemistryXylose
A composition, method of preparation1 and use to enhance the cosmetic feel of oil-in-water emulsions based upon the addition to the oil in water emulsion of one or more alkylpolyxylosides represented by formula:R—O—(X)p,wherein p is a decimal number between 1 and 5,wherein X is a xylose residue, andwherein R is a branched alkyl radical represented by the formula:CH(CnH2n+1)(CmH2m+1)—CH2—wherein m is an integer between 6 and 12, n is an integer between 8 and 16, and the sum of m+n is in the range of from about 14 to 26.
Owner:SOC DEXPLOITATION DE PROD POUR LES IND CHEM SEPPIC
Xylitol dehydrogenase of acetic acid bacteria and gene thereof
InactiveUS6924131B2Excellent in xylitol production abilityEfficient productionBacteriaSugar derivativesXylitol dehydrogenase activityA-DNA
Xylitol is produced by allowing xylitol dehydrogenase or cells instoduced with a DNA coding for xylitol dehydrogenase, which is a protein of the following (A) or (B) to act on D-xylulose, and collecting produced xylitol:(A) a protein which has the amino acid sequence of SEQ ID NO: 4;(B) a protein which has the amino acid sequence of SEQ ID NO: 4 including substitution, deletion, insertion, addition, or inversion of one or several amino acids, and has xylitol dehydrogenase activity.
Owner:AJINOMOTO CO INC
Application of flavone glycoside compounds in preparing medicament for treating and preventing hepatitis
InactiveCN102379888BImprove in vitro damageSignificant improvementOrganic active ingredientsDigestive systemApigeninGlucoside
The invention belongs to the field of pharmacy, and provides application of three flavone glycoside compounds luteolin-7-O-[alpha-L-rhamnose-(1->2)]-beta-D-glucoside (trivial name is lonicerin), apigenin-7-O-[alpha-L-rhamnose-(1->2)]-beta-D-glucoside (trivial name is rhoifolin) and luteolin-6-C-beta-D-glucose-8-C-beta-D-xyloside in preparing medicament or health-care food for preventing and treating hepatitis.
Owner:JIANGXI UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Method for extracting taxane active ingredients from Chinese yew efficiently
InactiveCN103408512BReduce usageReduce the difficulty of separation and purificationSugar derivativesSugar derivatives preparationOrganic solventBaccatin III
The invention discloses a method for extracting taxane type active ingredients from Chinese yew efficiently. The method comprises the following steps of: firstly extracting with water, further extracting with alcohol, and further purifying by a traditional purification method to get paclitaxel, 10-deacetyl baccatin III, taxol-7-xyloside, cephalomanine and other non-water-soluble taxane type substance monomers. According to the method disclosed by the invention, macroporous resin is applied to the extraction process, the extraction yield of the active ingredients is far higher than that of a traditional extraction method, the using quantity of an organic solvent is further significantly reduced, and the method is suitable for large-scale production of the paclitaxel and comprehensive extraction of other taxane active ingredient substances.
Owner:浙江大德龙生物技术有限公司
Taxus chinensis (Pilg.) Rehd. var. mairei (Lemee et Levl.) Cheng et L. K. Fu (T. speciosa Florin) formula granules, and preparation method and quality control method thereof
InactiveCN111135147AGuaranteed efficacySimple processAntipyreticComponent separationBiotechnologyBaccatin III
The invention discloses a preparation method for taxus chinensis (Pilg.) Rehd. var. mairei (Lemee et Levl.) Cheng et L. K. Fu (T. speciosa Florin) formula granules. The preparation method comprises the following steps of: after water is added into taxus chinensis (Pilg.) Rehd. var. mairei (Lemee et Levl.) Cheng et L. K. Fu (T. speciosa Florin) for dipping, heating to 50-70 DEG C, carrying out stirring and extraction for 1.5-5 h, collecting extracting solutions, adding water into residual medicine residues for repeated extraction, and combining the extracting solutions; after the obtained extracting solution is filtered, carrying out vacuum concentration to obtain a clear cream; drying the clear cream to obtain a dry cream; and after the dry cream is smashed and sieved, obtaining a dry cream powder, then, adding auxiliary materials, evenly mixing, and carrying out granulation. While 10-deacetylation Baccatin III and 7-xyloside-10-deacetylation taxol obtained by the preparation method have a high content transfer rate, a cream yield is relatively high, and 1g of the prepared taxus chinensis (Pilg.) Rehd. var. mairei (Lemee et Levl.) Cheng et L. K. Fu (T. speciosa Florin) granules isequivalent to 2.5g of taxus chinensis (Pilg.) Rehd. var. mairei (Lemee et Levl.) Cheng et L. K. Fu (T. speciosa Florin) decoction pieces. The invention also provides a control characteristic atlas ofthe taxus chinensis (Pilg.) Rehd. var. mairei (Lemee et Levl.) Cheng et L. K. Fu (T. speciosa Florin) granules, and a construction method for the control characteristic atlas, and is used for controlling the quality of the taxus chinensis (Pilg.) Rehd. var. mairei (Lemee et Levl.) Cheng et L. K. Fu (T. speciosa Florin) granules, specificity is good, and a repetitive rate is high and stable.
Owner:HUBEI XIANGRUIFENG ECOLOGICAL FRUIT IND CO LTD
Spiraea salicifolia stem and branch anti-rheumatoid-arthritis effective part and preparation method and application thereof
InactiveCN105796680AClear ingredientsHigh content of active ingredientsOrganic active ingredientsAntipyreticInterleukin 6Additive ingredient
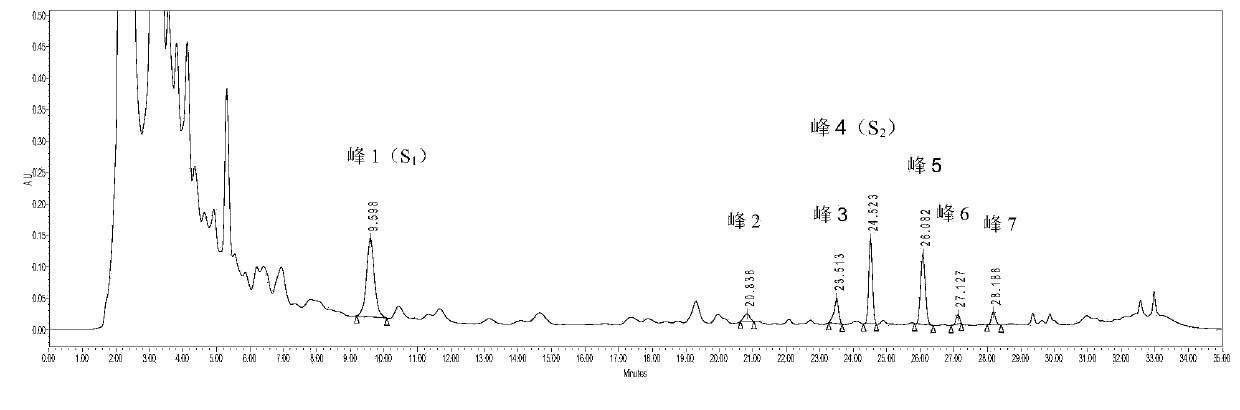
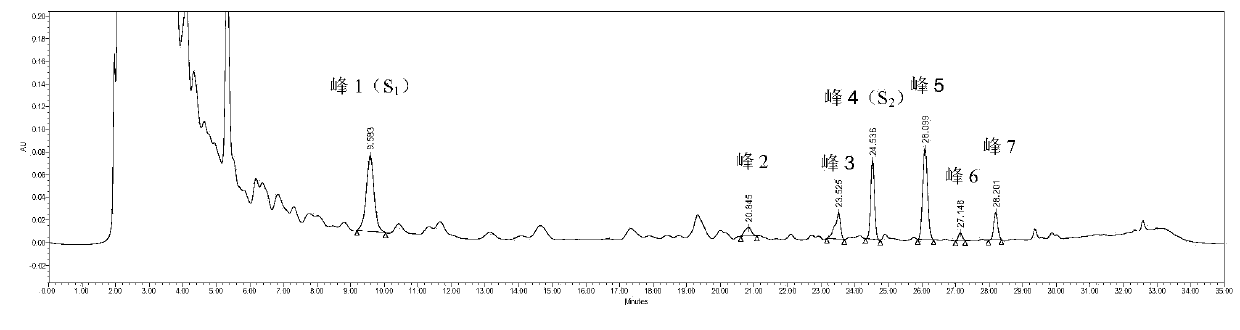
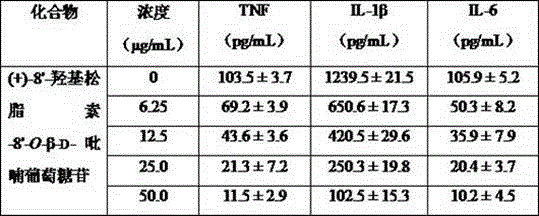
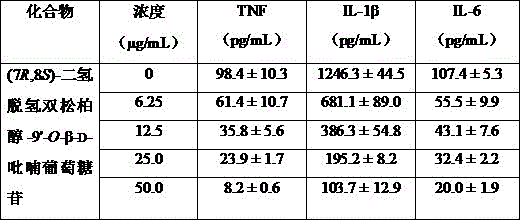
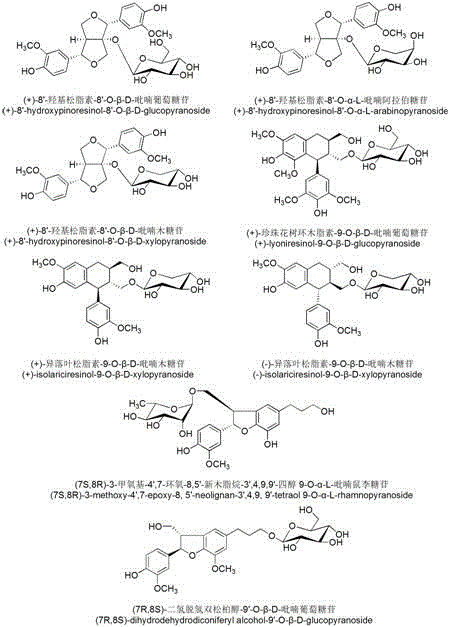
The invention provides a spiraea salicifolia stem and branch anti-rheumatoid-arthritis effective part and a preparation method and application thereof.According to the effective part, spiraea salicifolia stems and branches serve as raw materials, extract is obtained after 60% ethyl alcohol reflux extraction and ethyl alcohol pressure reduction recovery, the extract is purified with macroporous adsorption resin, and the total lignanoside effective part is obtained.The sum of weights of (+)-8'-hydroxy pinoresinol-8'-O-beta-d-glucopyranoside, (7R,8S)-dihydro dehydrogenation bis-coniferyl alcohol-9'-O-beta-d-glucopyranoside, (+)-lyonia ovalifolia tree ring lignans-9-O-beta-d-glucopyranoside and (+)-isolariciresinol-9-O-beta-d-pyran xyloside in the effective part ranges from 40% to 50%.The effective part is determined through the method that a modern separation means and pharmacological activity screening are combined, the ingredients of obtained effective part are clear, the content of active ingredients is high, it is shown through the in-vitro and in-vivo experiment results that the spiraea salicifolia stem and branch anti-rheumatoid-arthritis effective part inhibits generation of pro-inflammatory cytokine tumor necrosis factor-alpha, interleukin-1 beta and interleukin-6 to achieve the effect of treating rheumatoid arthritis.
Owner:QINGDAO UNIV
Liquid detergent with acarus killing effect
InactiveCN111440674AGood water solubilityResidue reductionNon-ionic surface-active compoundsOrganic detergent compounding agentsSuccinic acidDioxyethylene Ether
The invention discloses a liquid detergent with an acarus killing effect. The liquid detergent is prepared from the following raw materials in parts by weight: 5-12 parts of coconut oil fatty acid; 15-30 parts of a 40% sodium hydroxide aqueous solution; 30-40 parts of fatty alcohol-polyoxyethylene ether; 10-16 parts of 12-hydroxy-octadecanoic acid triglyceride; 4-8 parts of sodium p-toluenesulfonate, 3-8 parts of cocoanut fatty acid diethanolamide, 20-30 parts of an acarus killing composition, 1.5-3 parts of an enzyme preparation, 0.5-2 parts of succinic acid, 5-12 parts of EDTA, 0.2-0.5 partsof essence, 35-60 parts of propylene glycol and 150-200 parts of deionized water. The acarus killing composition is added into the detergent, and the prepared acarus killing xyloside derivative has good water solubility, high sterilization and acarus killing rate, no residue and good practical application prospect.
Owner:杭州中著智能科技开发有限公司
Crystal skin makeup primer with added hydrolyzed pearl, and preparation method of crystal skin makeup primer
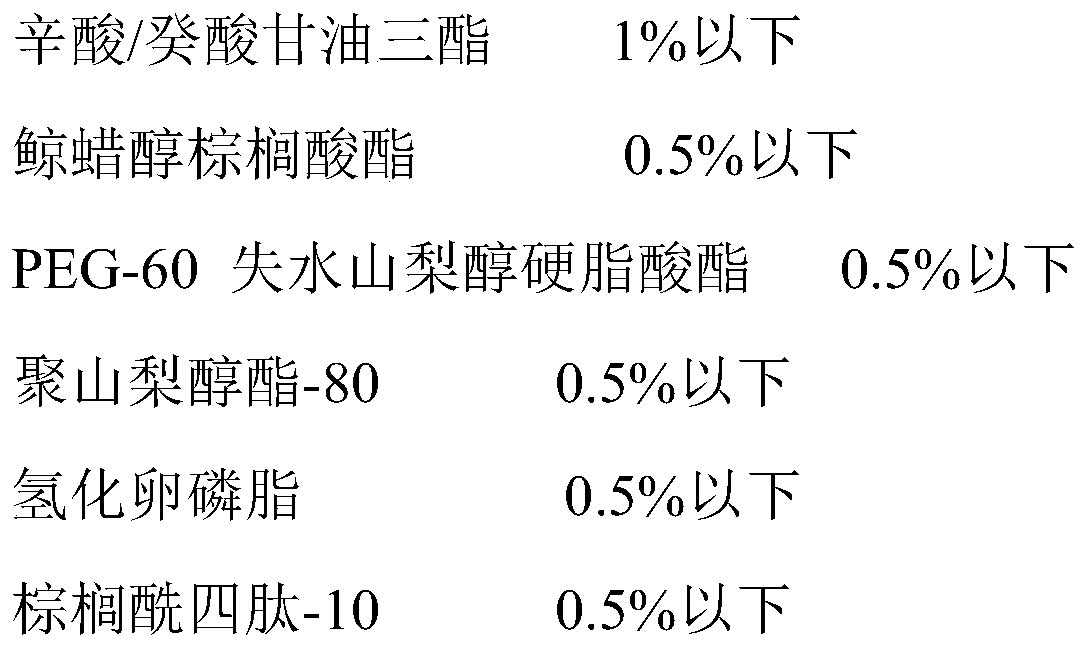
InactiveCN110384635ALow conductivityAvoid damageCosmetic preparationsToilet preparationsAdditive ingredientPalmitoyl tetrapeptide
The invention belongs to the technical field of daily cosmetics, and relates to crystal skin makeup primer with an added hydrolyzed pearl, and a preparation method of the crystal skin makeup primer. The preparation method comprises the steps that firstly, octyldodecanol, octyldodecyl xyloside, methyl hydrogenated rosinate and the like are heated and dissolved, then essence is added for even stirring, and thus a mixture is obtained for standby application; the hydrolyzed pearl, a hydroxyethyl acrylate / sodium acryloyldimethyl taurate copolymer, sorbitan isostearate, polysorbate-60, pure water and the like are added into an emulsifying pot, after stirring and heating, phenoxyethanol, ethylhexylglycerin, palmitoyl tetrapeptide-10 and the like are added, and even stirring is conducted; and themixture is slowly added into the emulsifying pot, after even mixing and stirring, high-speed homogenization is conducted, and after physicochemical indexes are checked out to be qualified, dischargingis conducted. The palmitoyl tetrapeptide-10 and the hydrolyzed pearl are synergistically compounded, the moisturizing degree of skin can be increased, the clarity of the skin can be improved, with assisting of skin filling ingredients, the skin is finer, uniform and bright, the skin is nourished from inside, and thus the skin can show the natural luster.
Owner:HAINAN JINGRUN PEARL BIOTECH
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com