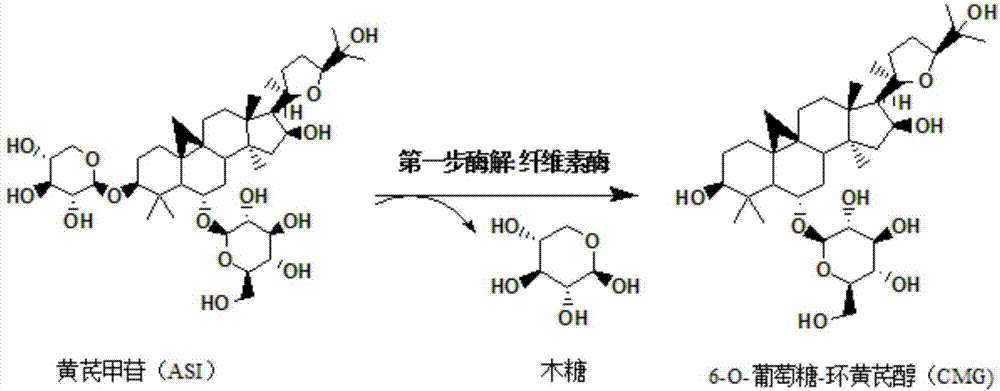
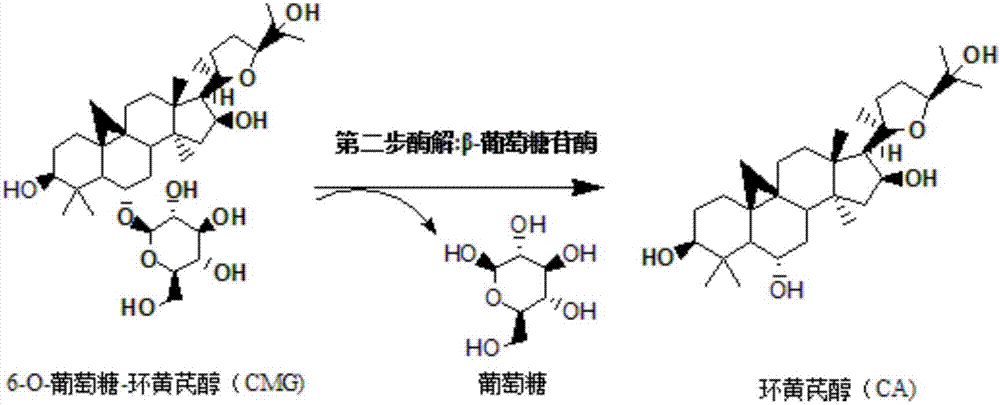
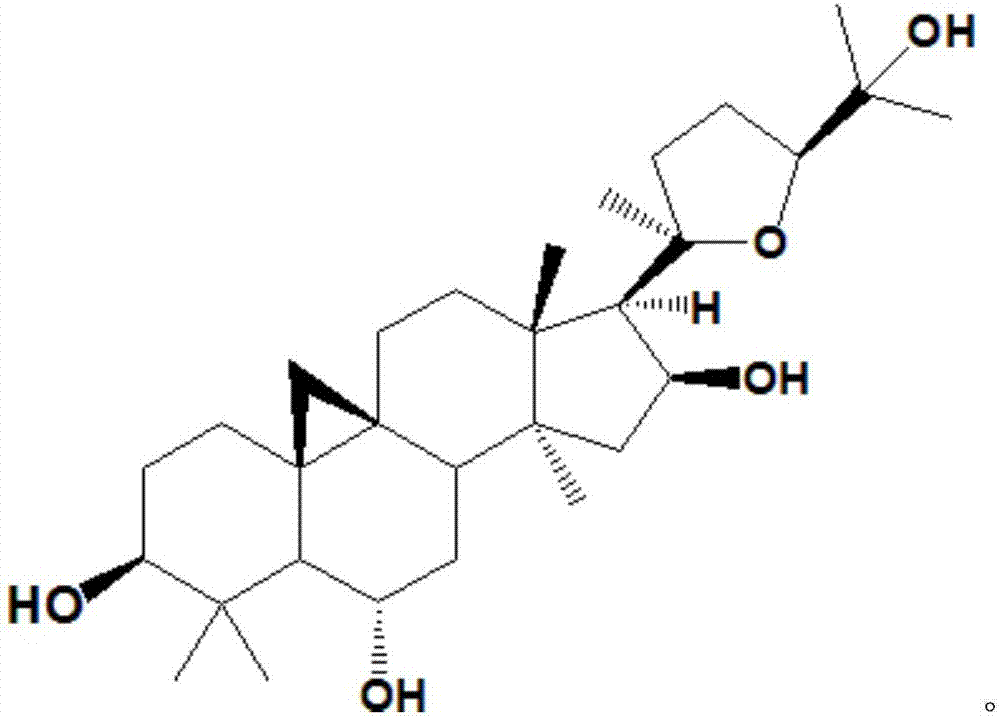
Method for converting astragalosides to prepare cycloastragenol by two-step enzymolysis
A technology of astragaloside IV and cycloastragenol, which is applied in the field of medicine and chemical industry, can solve the problems of three-membered rings that are easy to crack astragalol, and achieve the effects of reduced energy consumption, high purity, and mild preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Accurately prepare 0.2 mol / L disodium hydrogen phosphate solution and 0.1 mol / L citric acid solution, mix the two solutions at a volume ratio of 1.06:1, and adjust the pH of the buffered saline solution to 5.0 with hydrochloric acid.
[0036] The first step of enzymatic hydrolysis: accurately measure 100mL of enzymatic hydrolysis buffer saline solution and place it in a 250mL beaker, and add 100mg of astragaloside IV with a mass percentage of 10% to it, and heat the system after the substrate astragaloside IV is completely dissolved to 45°C, then add 0.05g of cellulase that has been heated to 45°C into the system, and adjust the pH of the system to 4.6 with hydrochloric acid, and finally place the reaction system on a magnetic heating and stirring instrument at a stirring speed of 200r / min , The temperature is 45 ℃ and fully reacted for 60h. Afterwards, 1 mL of the sample was passed through a 0.22 μm filter membrane and detected by high-performance liquid chromatography...
Embodiment 2
[0041] Accurately prepare 0.2 mol / L disodium hydrogen phosphate solution and 0.1 mol / L citric acid solution, mix the two solutions at a volume ratio of 1.06:1, and adjust the pH of the buffered saline solution to 5.0 with hydrochloric acid.
[0042] The first step of enzymatic hydrolysis: accurately measure 100mL of enzymatic hydrolysis buffer saline solution and place it in a 250mL beaker, and add 100mg of astragaloside IV with a mass percentage of 10% to it, and heat the system after the substrate astragaloside IV is completely dissolved to 50°C, then add 0.05g of cellulase that has been heated to 50°C into the system, and adjust the pH of the system to 5.0 with hydrochloric acid, and finally place the reaction system on a magnetic heating stirring instrument at a stirring speed of 200r / min , The temperature is 50 ℃ and fully reacted for 48h. Afterwards, 1 mL of the sample was passed through a 0.22 μm filter membrane and detected by high-performance liquid chromatography. Th...
Embodiment 3
[0047] Accurately prepare 0.2 mol / L disodium hydrogen phosphate solution and 0.1 mol / L citric acid solution, mix the two solutions at a volume ratio of 1.06:1, and adjust the pH of the buffered saline solution to 5.0 with hydrochloric acid.
[0048] The first step of enzymatic hydrolysis: accurately measure 100mL of enzymatic hydrolysis buffer saline solution and place it in a 250mL beaker, and add 100mg of astragaloside IV with a mass percentage of 10% to it, and heat the system after the substrate astragaloside IV is completely dissolved to 55°C, then add 0.05g of cellulase that has been heated to 55°C into the system, and adjust the pH of the system to 5.5 with hydrochloric acid, and finally place the reaction system on a magnetic heating and stirring instrument at a stirring speed of 200r / min , The temperature is 55 ℃ and fully reacted for 72h. Afterwards, 1 mL of the sample was passed through a 0.22 μm filter membrane and detected by high-performance liquid chromatography...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com