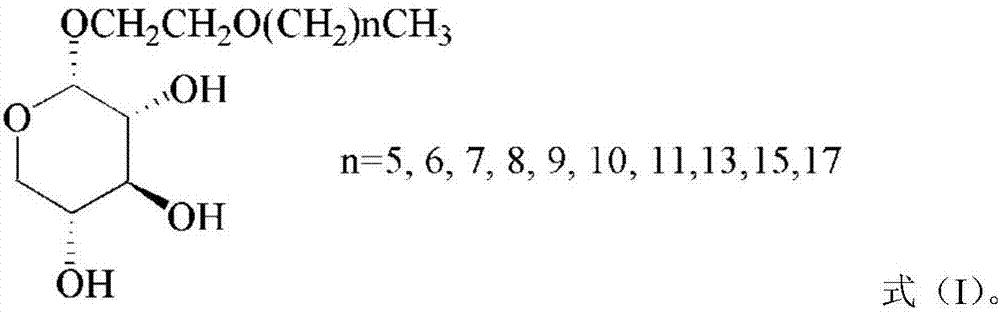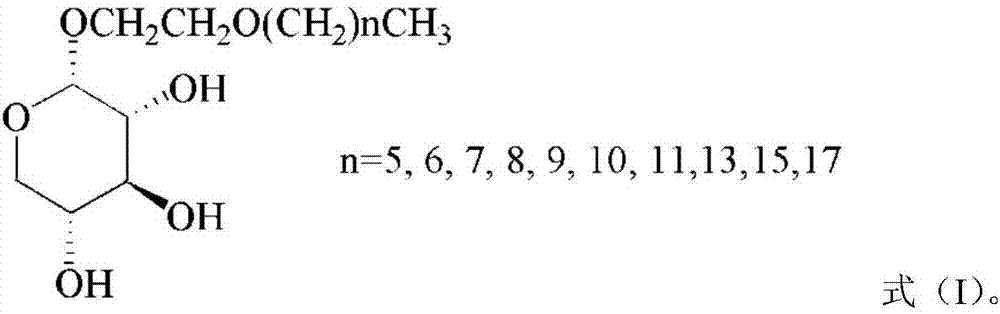Novel 1,2-cis-xyloside surfactant
An active agent, xyloside technology, applied in the field of sugar-based surfactants, alkoxyethyl-α-D-xylopyranoside surfactants, can solve the problem of water solubility and surface activity, and it is difficult to achieve surface activity The application value of the agent, the decrease of water solubility, etc., can achieve the effect of optional controllable foaming performance and emulsification performance, good market prospect and application value, and improvement of water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
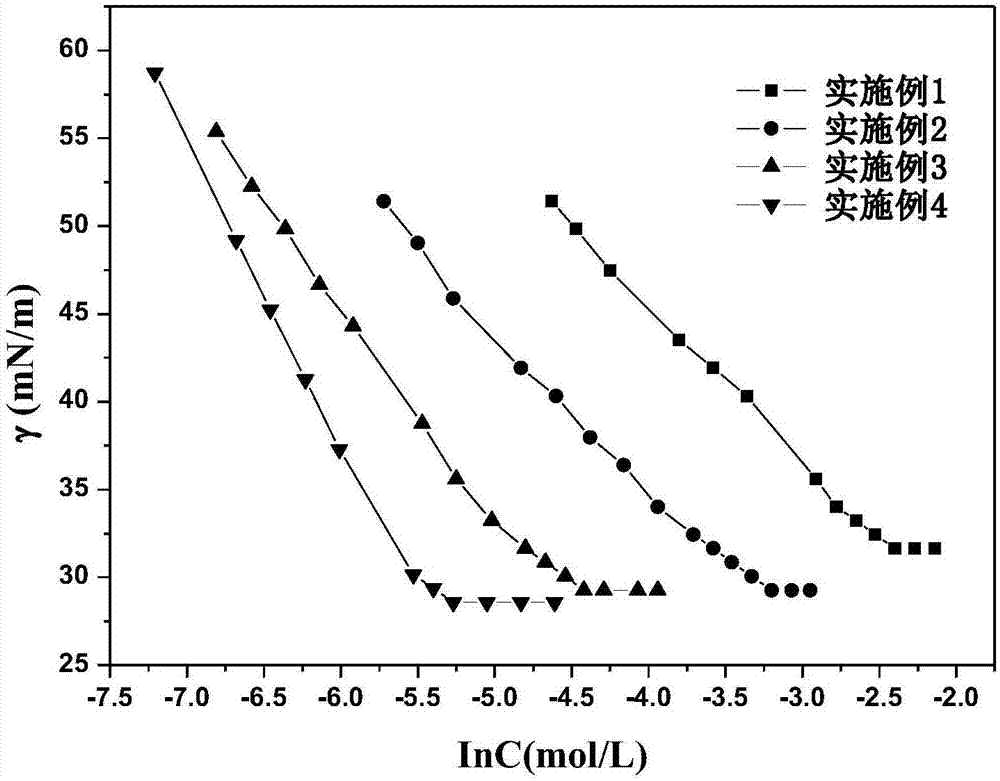
Image
Examples
preparation example Construction
[0025] On the basis of providing alkoxyethyl-α-D-xylopyranoside with novel structure, the present invention also provides a preparation method, comprising the following steps:
[0026] (1) In the presence of a catalyst, D-xylose is contacted with a protecting agent to obtain acyl-protected D-xylose;
[0027] (2) In the presence of a catalyst, contact the acyl-protected D-xylose obtained in step (1) with ethylene glycol monoalkyl ether to obtain a 1,2-cis coupling product acyl-protected alkoxy Ethyl-α-D-xylopyranoside;
[0028] (3) In the presence of a catalyst, the 1,2-cis acyl-protected alkoxyethyl-α-D-xylopyranoside obtained in step (2) is subjected to deacyl protection to obtain 1,2- The structure of cis-alkoxyethyl-α-D-xylopyranoside is shown in formula (I).
[0029]
[0030] According to a preferred embodiment of the present invention, in step (1), the catalyst is anhydrous sodium acetate, and the protective agent is acetic anhydride, and the following reaction formu...
example 1
[0046] Example 1: Preparation of Hexyloxyethyl-α-D-xylopyranoside
[0047] (1) Add 0.13mol of dried D-xylose, 0.67mol of acetic anhydride, and 36.59mmol of anhydrous sodium acetate to a 250mL three-necked flask in turn, install a reflux condensing device, stir mechanically, and heat up with an electric heating mantle to make the solid slightly After dissolving, remove the heating device and continue to stir until all the solids are dissolved and become clear, then cool to room temperature. Then add 30mmol anhydrous sodium acetate, move the device into an oil bath, heat to reflux for 1h, TLC (developing agent: V 石油醚 :V 乙酸乙酯 =1:1) Monitor the complete reaction, pour the reaction solution into 400mL of ice water while it is hot and stir, then precipitate a large amount of solid, filter with suction, and wash the filter cake with distilled water several times to obtain 35.02g of acetyl-protected D-xylose, producing The rate is 82.6%. With methanol solution (V 甲醇 :V 水 =1:2) re...
example 2
[0051] Example 2: Preparation of Heptyloxyethyl-α-D-xylopyranoside
[0052] (1) With step (1) in embodiment 1.
[0053] (2) In a 250mL round bottom flask, add 31.42mmol of the acetyl-protected D-xylose obtained in step (1), dissolve with an appropriate amount of dichloromethane, then add 47.16mmol of ethylene glycol monoheptyl ether, and Boron trifluoride diethyl ether 0.16mol was added dropwise under the bath, magnetically stirred for 9 hours, TLC (developing solvent: V 石油醚 :V 乙酸乙酯 =5: 1) The detection reaction is complete, the mixed solution is washed successively with saturated aqueous sodium bicarbonate solution and saturated saline solution, dried with anhydrous sodium sulfate, filtered, the filtrate is concentrated, and column chromatography (V 石油醚 :V 乙酸乙酯 =10: 1) separation to obtain 5.26 g of heptyloxyethyl-2,3,4-tri-O-acetyl-α-D-xylopyranoside with a yield of 40.0%. used directly in the next reaction.
[0054] (3) Add 12.57mmol of heptyloxyethyl-2,3,4-tri-O-acety...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com