Detection method for fingerprint spectrum of medicinal preparation treating hepatitis
A technology of pharmaceutical preparations and detection methods, which is applied in the field of fingerprint detection, and can solve the problems of time-consuming, difficult production practice and laborious joint detection of multiple detection indicators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The raw materials of Yindan Pinggan Capsules used in the following examples and experimental examples are as follows: capillary 350g, gentian 550g, Scutellaria baicalensis 75g, porcini powder 125g, gardenia 75g, peony root 125g, angelica 75g, licorice 125g ;
[0059] Prepare according to the following preparation method: take pig bile powder and add 3 times the amount of water to dissolve, add hydrochloric acid and heat to boil to acidify, let it cool, add water to precipitate the precipitate, add 0.8 times the amount of solid sodium hydroxide and 4.5 times the amount of water to dissolve the precipitate, Heating and saponifying for 10 hours, standing overnight, adding 5.5 times the amount of hydrochloric acid to acidify, letting cool, precipitated, filtered, washed the precipitate with water until neutral, dried, and crushed into fine powder to obtain refined pig gallbladder powder;
[0060] Take slices of Angelica sinensis, soak overnight with 4.5 times the amount of ...
Embodiment 2
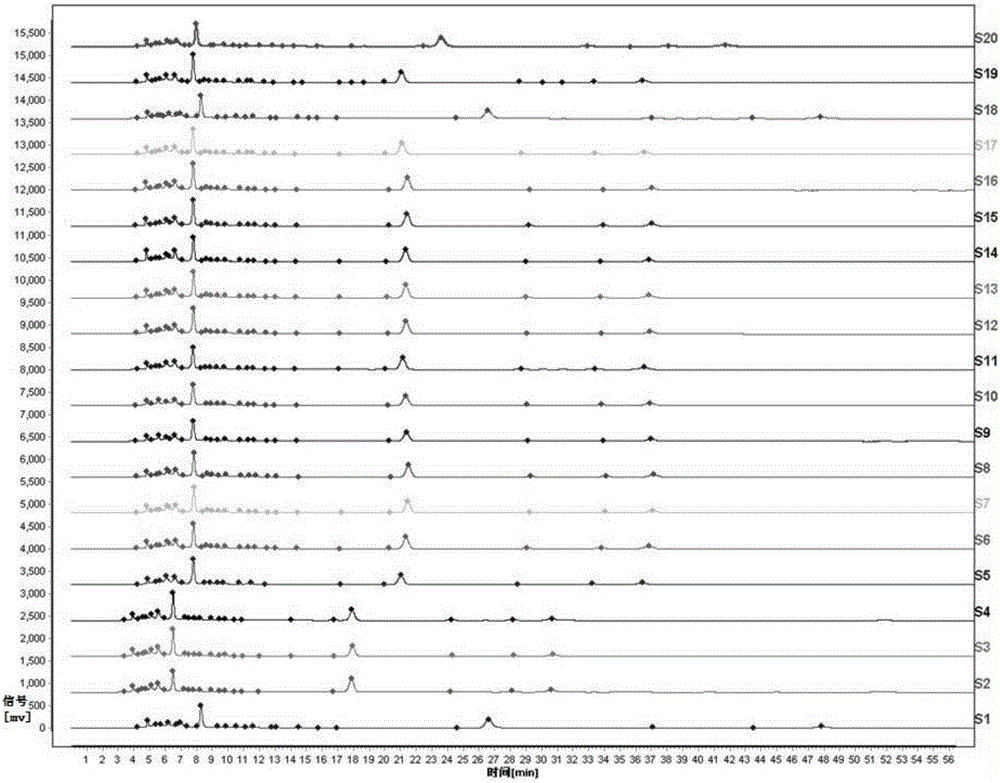
[0063] The detection method of the fingerprint of the present embodiment Yindan Pinggan Capsules comprises the following steps:
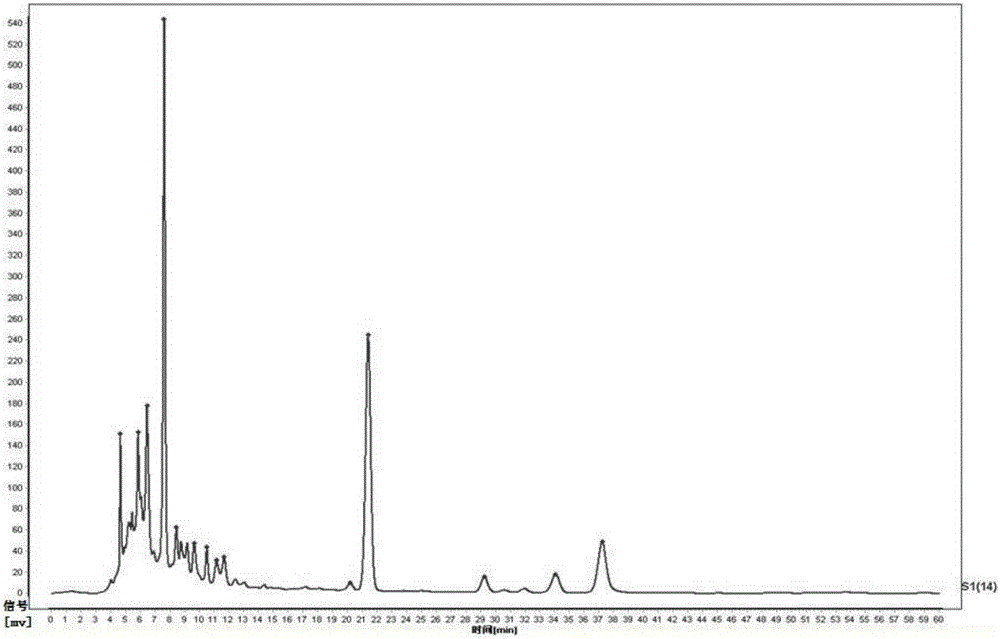
[0064] (1) Take 10 g of the drug preparation to be tested, grind it finely, take 2 g, accurately weigh it, place it in a stoppered Erlenmeyer flask, add 50 mL of 50% methanol solution accurately, weigh it, heat and reflux for extraction for 30 minutes, let it cool, and then Weigh the weight, take 50% methanol solution to make up the lost weight, shake well, filter, and get the filtrate as the test solution;
[0065] (2) Accurately weigh the baicalein reference substance, add methanol to make a solution containing 0.00009g per 1mL, shake well, and use it as the reference substance solution;
[0066] (3) Chromatographic conditions: Octadecylsilane bonded silica gel is used as filler, methanol-0.1% phosphoric acid mixed solution with a volume ratio of 55:45 is used as mobile phase, the detection wavelength is 274nm, and the number of theoretical plates...
Embodiment 3
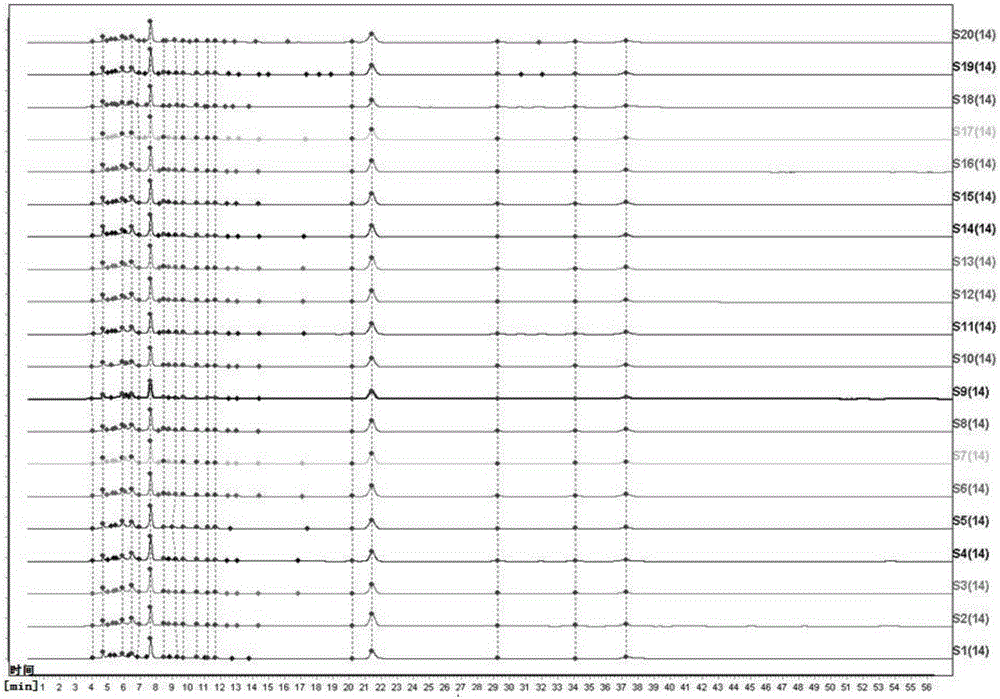
[0070] The detection method of the fingerprint of the present embodiment Yindan Pinggan Capsules comprises the following steps:
[0071] (1) Take 5 g of the pharmaceutical preparation to be tested, grind it finely, take 1 g, accurately weigh it, place it in a stoppered Erlenmeyer flask, add 40 mL of 40% methanol solution accurately, weigh it, heat and reflux for extraction for 20 minutes, let it cool, and then Weigh the weight, take 40% methanol solution to make up the lost weight, shake well, filter, and get the filtrate as the test solution;
[0072] (2) Accurately weigh the baicalein reference substance, add methanol to make a solution containing 0.00005g per 1mL, shake well, and use it as the reference substance solution;
[0073] (3) Chromatographic conditions: Octadecylsilane bonded silica gel is used as filler, methanol-0.1% phosphoric acid mixed solution with a volume ratio of 50:50 is used as mobile phase, the detection wavelength is 274nm, and the number of theoretic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com