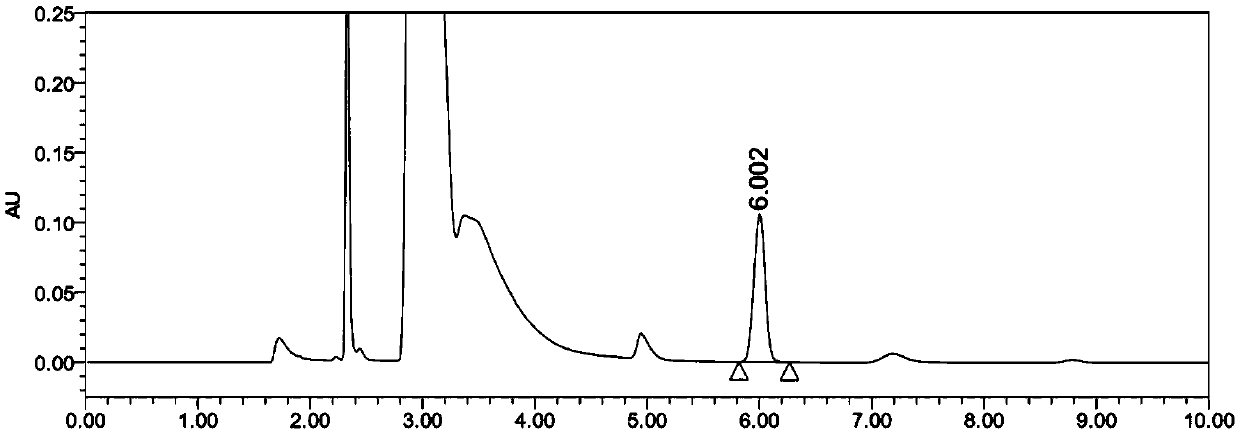
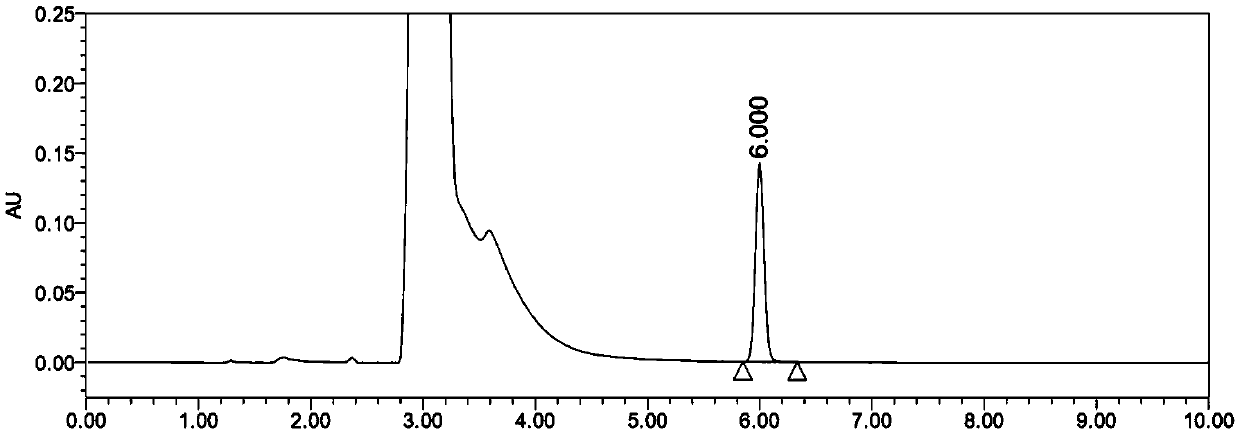
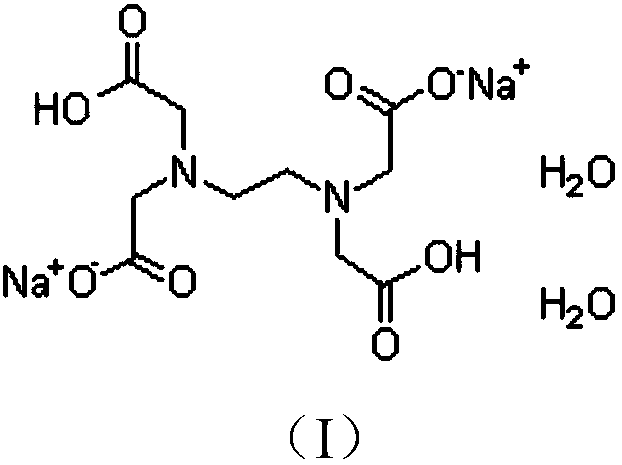
Content determination method of edetate disodium in acetylcysteine liquid preparation
A technology of acetylcysteine and disodium edetate, applied in the field of content determination of disodium edetate, can solve the interference of determination, cannot meet the requirements of content determination, cannot meet the requirements of accurate quantification of disodium edetate, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] 1. Chromatographic conditions:
[0075] Chromatographic column: Aminosilane bonded silica gel as filler, Agilent 4.6×250mm, filler particle diameter 5μm;
[0076] Column temperature: 25°C;
[0077] Mobile phase: a solution containing 0.1% tetradecyl ammonium bromide and 0.05% tetraheptyl ammonium bromide (phosphoric acid to adjust the pH to 2.0)-methanol (volume ratio 95:5) as the mobile phase;
[0078] Flow rate: 2mL / min;
[0079] Injection volume: 5μL;
[0080] Detection wavelength: 240nm.
[0081] 2. Preparation of test solution:
[0082] (1) Preparation of the test solution: accurately measure 0.2 mL of acetylcysteine solution for inhalation, put it in a 10 mL measuring bottle, add 20 mg of hydroxypropyl-β-cyclodextrin, and then add 0.5% ferric chloride solution 0.4mL, and finally add mobile phase to dilute to the mark, shake well, and filter to obtain the test solution.
[0083] (2) Preparation of reference solution: Take 25 mg of edetate disodium, accurate...
Embodiment 2
[0087] 1. Chromatographic conditions:
[0088] Chromatographic column: Aminoalkylsilane bonded silica gel as filler, Agilent 4.6×250mm, filler particle diameter 5μm; column temperature: 35°C;
[0089] Mobile phase: a solution containing 2% tetradecyl ammonium bromide and 1% tetraheptyl ammonium bromide (phosphoric acid to adjust the pH to 3.0)-methanol (volume ratio 70:30) as the mobile phase;
[0090] Flow rate: 0.5mL / min;
[0091] Injection volume: 50μL;
[0092] Detection wavelength: 280nm.
[0093] 2. Preparation of test solution:
[0094] (1) Preparation of the test solution: Accurately measure 2 mL of acetylcysteine solution for inhalation, put it in a 10 mL measuring bottle, add 200 mg of hydroxypropyl-β-cyclodextrin, and then add 4 mL of 0.5% ferric chloride solution , and finally add mobile phase to dilute to the mark, shake well, and filter to obtain the test solution.
[0095] (2) Preparation of reference solution: Take 25 mg of edetate disodium, accurately wei...
Embodiment 3
[0099] 1. Chromatographic conditions:
[0100] Chromatographic column: Aminoalkylsilane bonded silica gel as filler, Agilent 4.6×250mm, filler particle diameter 5μm;
[0101] Column temperature: 30°C;
[0102] Mobile phase: a solution containing 0.2% tetradecyl ammonium bromide and 0.1% tetraheptyl ammonium bromide (phosphoric acid to adjust the pH to 1.5)-methanol (volume ratio 90:10) as the mobile phase;
[0103] Flow rate: 1mL / min;
[0104] Injection volume: 20μL;
[0105] Detection wavelength: 257nm.
[0106] 2. Test solution preparation:
[0107] (1) Preparation of the test solution: accurately measure 1 mL of acetylcysteine solution for inhalation, put it in a 10 mL measuring bottle, add 100 mg of hydroxypropyl-β-cyclodextrin, and then add 2 mL of 0.5% ferric chloride solution , and finally add mobile phase to dilute to the mark, shake well, and filter to obtain the test solution.
[0108] (2) Preparation of reference solution: Take 25 mg of edetate disodium, acc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com