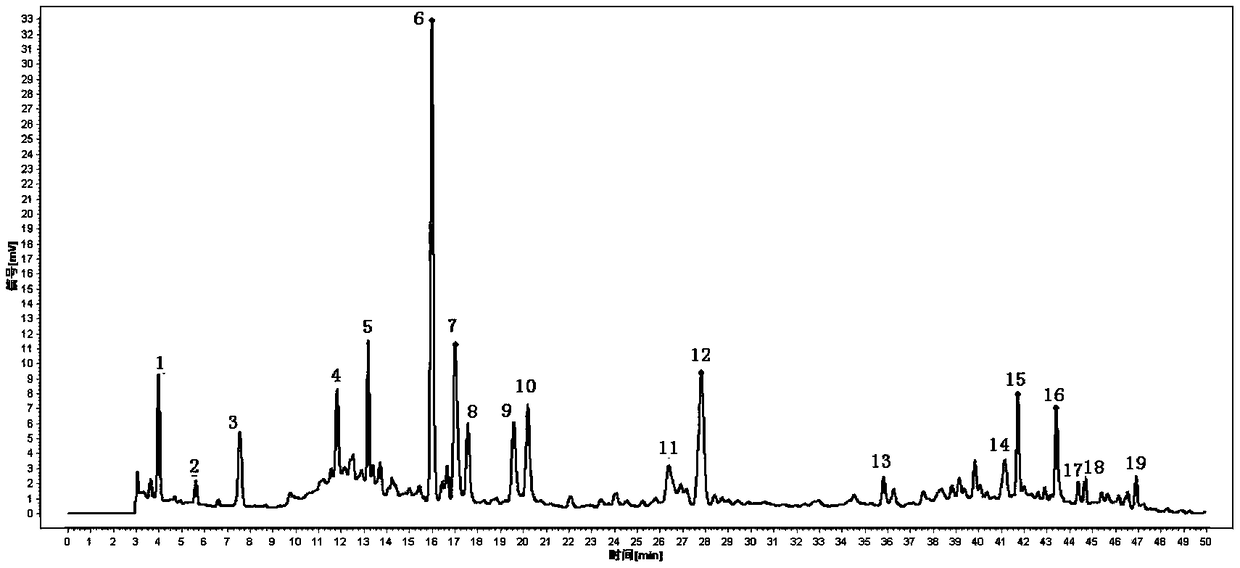
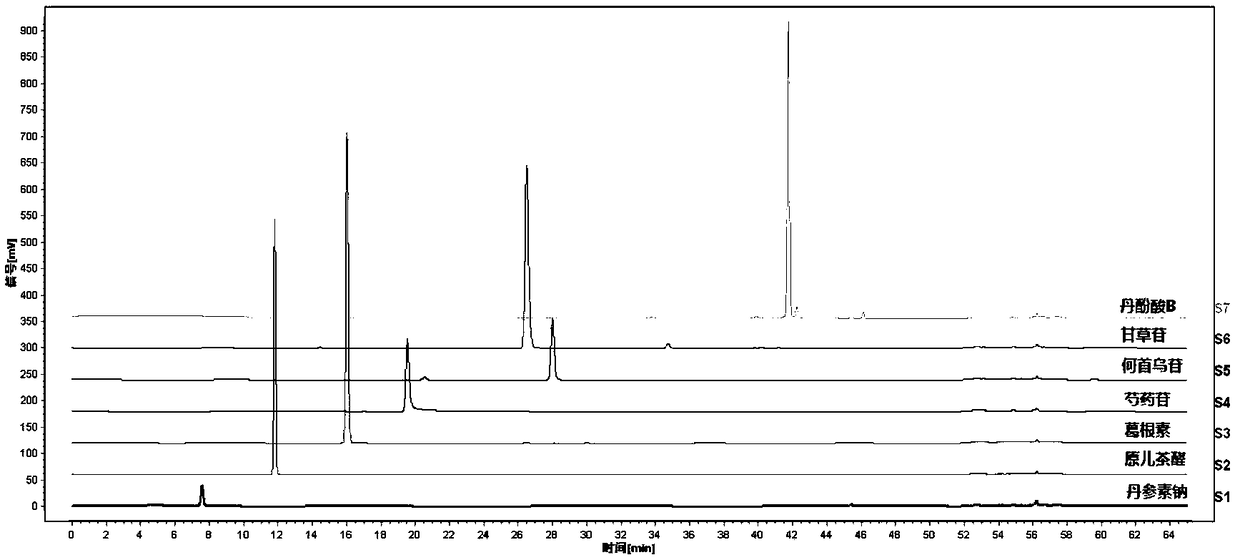
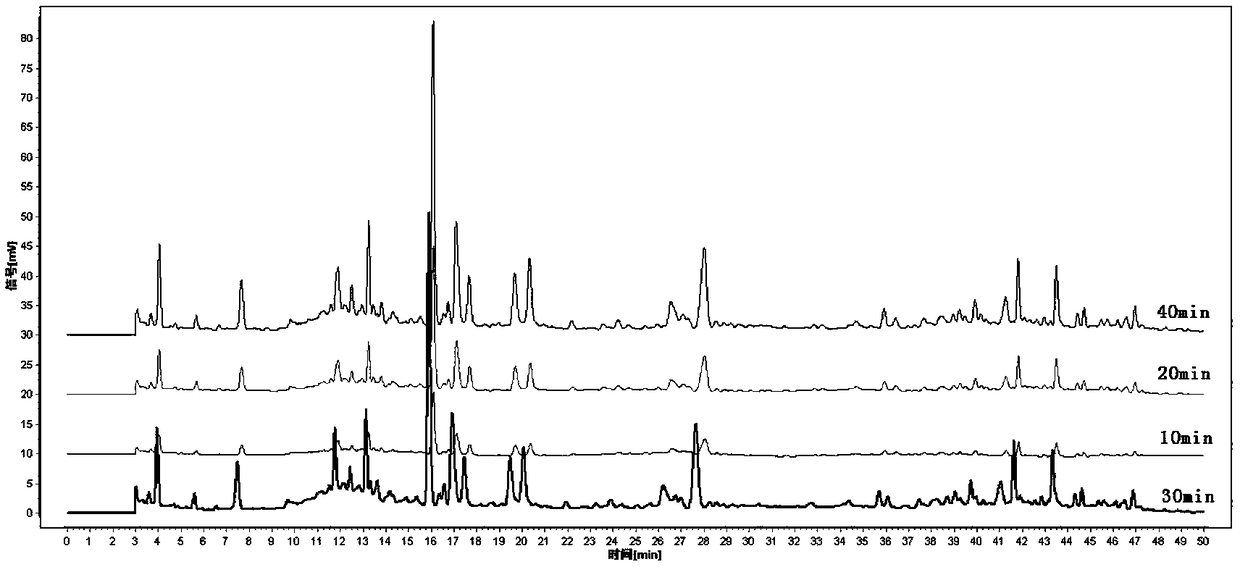
Determination method for fingerprint spectrum of Linaoxin Pian as well as standard fingerprint spectrum of Linaoxin Pian
A technology of Naoxin Tablet and its detection method, which is applied in the determination of HPLC fingerprints of Linaoxin Tablets and the field of fingerprints of Linaoxin Tablet preparations, can solve problems such as difficult to effectively control the overall quality, and cannot guarantee the safety and effectiveness of medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Step (1) The preparation method of the test solution is: take Linaoxin tablets, remove the coating, grind finely, weigh 1g, put in a stoppered Erlenmeyer flask, add 20ml of 50% methanol precisely, seal it tightly, and weigh it , sonicated for 30 minutes, allowed to cool, and then weighed, supplemented with the extraction solvent to make up the lost weight, filtered with a 0.45 μm microporous membrane, and the filtrate was the test solution.
[0101] Step (2) The concentration of the reference substance solution preparation is respectively: the concentration of the Danshensu reference substance is 55.8 μg / ml, the concentration of the protocatechualdehyde reference substance is 52.6 μg / ml, the concentration of the puerarin reference substance is 55.3 μg / ml, and the concentration of the peony reference substance is 55.8 μg / ml. The concentration of the glucoside reference substance was 27.6 μg / ml, the concentration of the polyglutinin reference substance was 25.5 μg / ml, the ...
Embodiment 2
[0108] For the fingerprint determination of batch 171202 of Linaoxin Tablets, the specific steps are as follows:
[0109] Step (1) The preparation method of the test solution is: take Linaoxin Tablet 171202, remove the coating, grind finely, weigh 1g, put it in a stoppered Erlenmeyer flask, add 20ml of double-distilled water precisely, seal it, and weigh it. Weight, sonicate for 30 minutes, let cool, weigh again, make up the lost weight with extraction solvent, filter with 0.45 μm microporous membrane, and the subsequent filtrate is the test solution.
[0110] Step (2) The concentration of the reference substance solution preparation is respectively: the concentration of the Danshensu reference substance is 55.8 μg / ml, the concentration of the protocatechualdehyde reference substance is 52.6 μg / ml, the concentration of the puerarin reference substance is 55.3 μg / ml, and the concentration of the peony reference substance is 55.8 μg / ml. The concentration of the glucoside referen...
Embodiment 3
[0118] For the fingerprint determination of batch 180303 of Linaoxin Tablets, the specific steps are as follows:
[0119] Step (1) The preparation method of the test solution is: take Linaoxin Tablet 180303, remove the coating, grind finely, weigh 1g, put it in a stoppered Erlenmeyer flask, add 20ml of double-distilled water precisely, seal it, and weigh it. Weight, sonicate for 30 minutes, let cool, weigh again, make up the lost weight with extraction solvent, filter with 0.45 μm microporous membrane, and the subsequent filtrate is the test solution.
[0120] Step (2) The concentration of the reference substance solution preparation is respectively: the concentration of the Danshensu reference substance is 55.8 μg / ml, the concentration of the protocatechualdehyde reference substance is 52.6 μg / ml, the concentration of the puerarin reference substance is 55.3 μg / ml; The concentration of the glucoside reference substance was 27.6 μg / ml; the concentration of the Polygonum glycos...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com