Establishment method of hplc fingerprint of Zhuang medicinal material Diangui Ainaxiang
A technology of fingerprints and establishment methods, which is applied in the field of establishment of HPLC fingerprints of Zhuang medicinal material Diangui Ainaxiang. The number of identifiable components is small, and the effects of improving fingerprint identification ability, perfect quality control and good reproducibility of scientific methods are achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1 Experimental materials and instruments
[0028] 1.1 Medicinal materials: place of origin and harvest time are shown in Table 1.
[0029] Table 1 The origin and harvest time of the medicinal materials of Yunnan Gui Ainaxiang
[0030]
[0031]
[0032] 1.2 Instrument: Agilent1100 high performance liquid chromatography (including G1311A quaternary pump, G1313A autosampler, G1314AVWD detector, G1316A column thermostat, Agilengt1100 chromatographic workstation (Agilent Technologies, USA)) Electronic analytical balance: BP211D (Sartorie, Germany Sri Lanka); PS-60AL Ultrasonic Cleaner (40kHz, 360W); "Chinese Medicine Chromatographic Fingerprint Similarity Evaluation System 2004 A Edition (National Pharmacopoeia Commission).
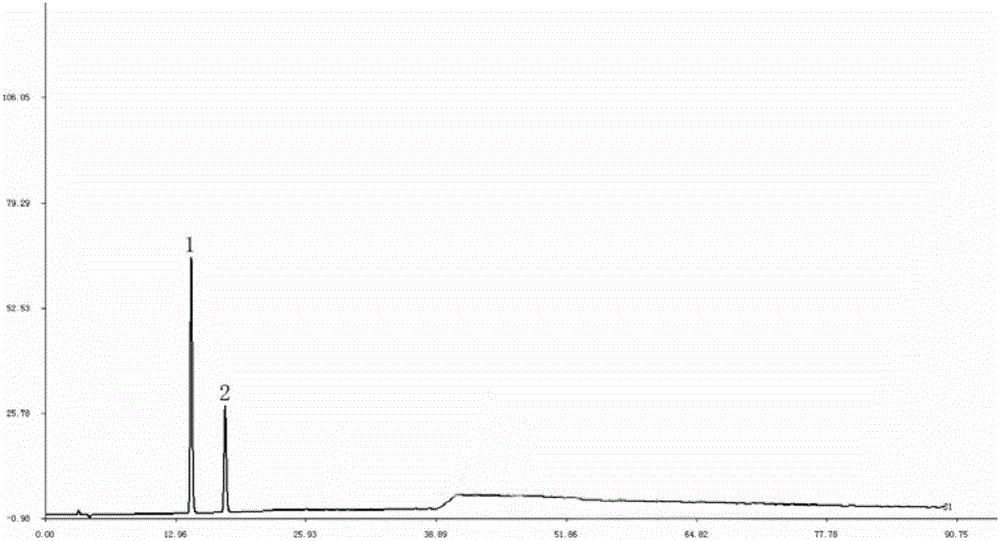
[0033] 1.3 Reagents: protocatechuic acid reference substance (purchased from China Institute for the Control of Pharmaceutical and Biological Products) batch number 110809-200604; protocatechualdehyde reference substance (purchased from China Ins...
Embodiment 2
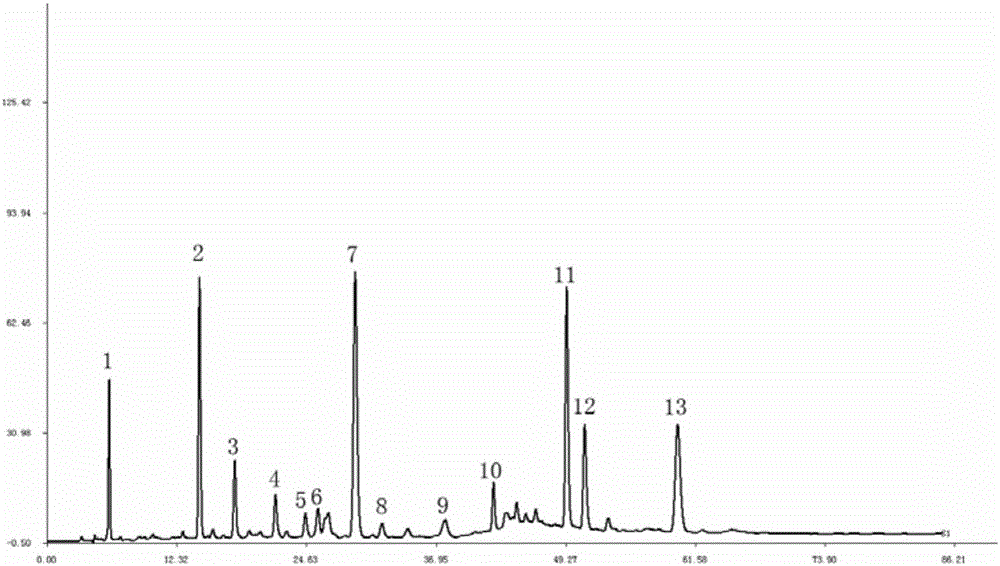
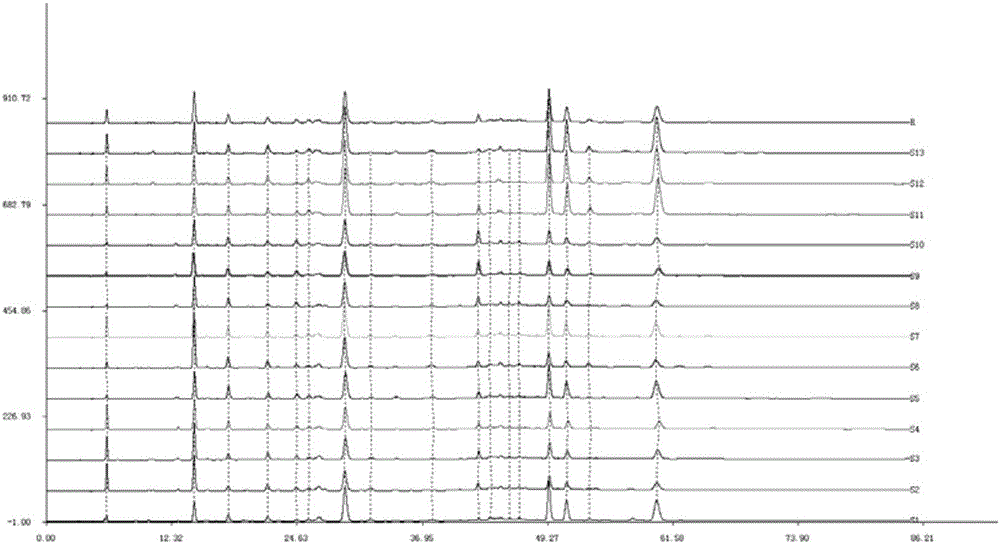
[0043] Take 13 batches of Zhuang medicine Diangui Ainaxiang medicinal materials, detect according to the conditions of Example 1, and obtain the overlay of the HPLC fingerprints of 13 batches of samples, as shown in image 3 shown. By comparing these 13 batches of HPLC chromatograms, carry out acquaintance evaluation, determine its characteristic common peak: there are 13 characteristic common peaks in the fingerprint chromatogram, its retention time, the average value of peak area and the ratio of total peak area are summarized as follows:
[0044] Peak No. 1 has an average retention time of 5.919min, an RSD% of 0.23%, a peak area of 294.446, and an RSD% of 70.14%, accounting for 4.027% of the total peak area;
[0045] Peak No. 2 has an average retention time of 14.515min, an RSD% of 0.12%, a peak area of 960.173, and an RSD% of 26.12%, accounting for 13.131% of the total peak area;
[0046] Peak No. 3 has an average retention time of 17.867min, an RSD% of 0.08%, a peak ...
Embodiment 3
[0063] 1. Methodological investigation
[0064] 1.1 Precision experiment
[0065] Get numbered and be No. 6 need testing sample, prepare by need testing solution preparation method in embodiment 1, record fingerprint spectrum under above-mentioned chromatographic condition. The results showed that the relative retention time RSD values of each chromatogram were 0.068%-0.204%, and the relative peak area RSD values of each peak were 0.045-1.76%. It shows that the precision of the instrument is good.
[0066] 1.2 Stability experiment
[0067] Get numbered and be No. 6 need testing sample, prepare by embodiment 1 need testing solution preparation method, under above-mentioned chromatographic condition, detect fingerprint at 0,4,8,12,16,24h respectively, investigate the repetition of experimental method sex. The results showed that the relative retention time RSDs of the 6 samples and chromatographic peaks were in the range of 0.038-0.336%, and the relative peak area RSD va...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com