Quality control method of Fuzi Lizhong Wan
A technology of aconite theory and detection method, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., to achieve the effect of strengthening uniformity and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 Fuzi Lizhong Pill Dissolution Quality Control Method
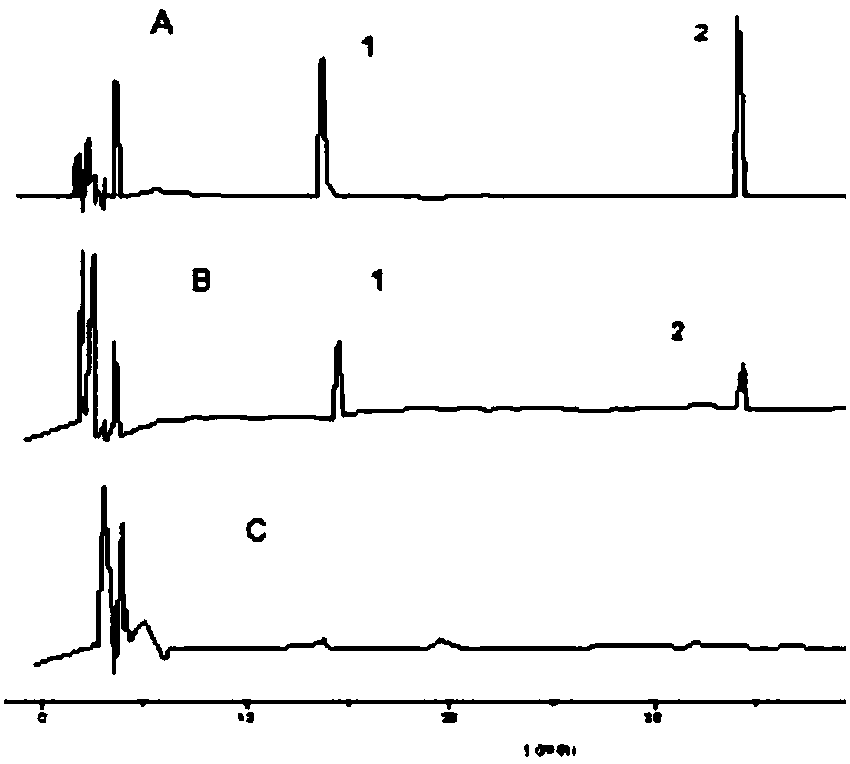
[0032] (1) Dissolution operation: connect the power of the dissolution apparatus, measure 900ml of 0.25% sodium lauryl sulfate newly prepared and ultrasonically placed in each dissolution cup, and wait for the dissolution medium (dissolution medium is water, 0.25% dodecane) Sodium sulfate solution, 0.5% hydrochloric acid water solution or phosphate buffer solution with pH6.8) After the temperature is kept constant at 37±0.5°C, set the rotation speed at 100 rpm, take 6 pills of Fuzi Lizhong Pills, and put them into 6 dissolution cups respectively Inside, close the lid tightly, start the propeller immediately and count the time; at 0.5, 1, 1.5, 2, 4, 12, 24, 48h, take 2mL of dissolution liquid (add 2ml of dissolution medium), and quickly use 0.22μm microporous Filter through a membrane to obtain the test solution.
[0033] (2) Determination of dissolution rate:
[0034] Chromatographic conditions: the c...
experiment example 1
[0044] Experimental example 1 evaluates the quality of commercially available Fuzi Lizhong Pills by the inventive method
[0045] (1) Instruments and reagents:
[0046] ZRS-8G Intelligent Dissolution Apparatus (Tianjin Tianda Tianfa Technology Co., Ltd.), METTLERTOLEDOPH Detector, ARSS4CN1 / 10 Analytical Balance (Ohaus Instruments Co., Ltd.), ARSS4CN1 / 1 Analytical Balance (Ohaus Instruments Co., Ltd.) , DL-720D Ultrasonic Machine (Shanghai Zhixin Instrument Co., Ltd.), Agilent-1260 High Performance Liquid Chromatography System (Agilent Corporation, USA), Chromatographic Column Agilent HC-C18 (250×4.6mm, 5.0μm);
[0047] Glycyrrhizic acid reference substance (Chengdu Pusi Biotechnology Co., Ltd. 1050-0025), liquiritin reference substance (Chengdu Pusi Biotechnology Co., Ltd. 161213-04), commercially available Fuzi Lizhong Pills (concentrated pills) batch number: manufacturer 1: 1605081, 1602053, 1605052, manufacturer 2: 1605211, 1605201, 1609231, manufacturer 3: 81151215, 81140...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com