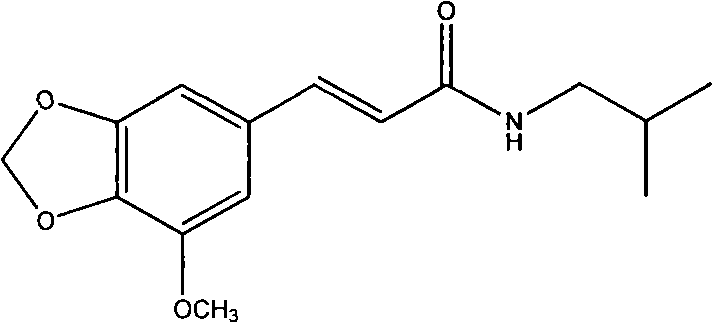
Application of 5'-methoxy-3',4'-methylenedioxy cinnamic acid isobutyl amide in preparing antidepressant medicaments
A technology of cinnamic acid isobutylamide and methylenedioxy, applied in the preparation of antidepressant drugs, in the field of amide compounds, can solve problems such as difficult to obtain satisfactory curative effect, no antidepressant drugs, emotional depression, depression, etc. , to achieve good therapeutic and preventive effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
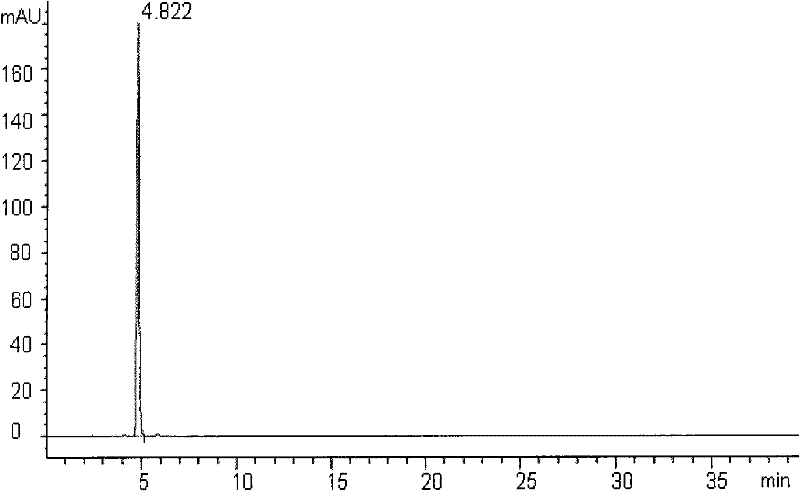
[0157] Embodiment 1 Preparation of compound G-1 of the present invention
[0158]Take the above-ground part of the betel leaf, crush it, add 8 times the amount of 95% ethanol, reflux extraction twice, each time for 2 hours, filter, combine the extracts, and concentrate under reduced pressure at 60°C to a paste. The extract is dissolved with 40% ethanol, separated through a macroporous adsorption resin column (crude drug-resin ratio 3:1), removed with 50% ethanol, then eluted with 95% ethanol, and the eluate is collected and concentrated to a paste. The extract was mixed with 100-200 mesh silica gel, separated by column according to the dry weight of the sample-silica gel ratio 1:30, and eluted successively with petroleum ether-ethyl acetate (5:1 and 3:1) as the mobile phase. Thin-layer chromatography detection, combined the same parts, to get G-1 ~ G-13, wherein the G-1 part was recrystallized with ethanol-water to get the monomer, compound G-1 (ie: compound 5'-methoxy- 3',4'...
Embodiment 2
[0159] The preparation of embodiment 2 tablet
[0160] G-1 20g
[0161] Microcrystalline Cellulose 50g
[0162] Lactose 50g
[0163] Starch 51g
[0164] Sodium carboxymethyl starch 12g
[0165] 5% PVP absolute ethanol appropriate amount
[0166]
[0168] Made into 1000 pieces
[0169] G-1 and other excipients in the prescription were passed through a 100-mesh sieve, and the prescription amount of G-1 was weighed, mixed with microcrystalline cellulose, starch and sodium carboxymethyl starch, and mixed evenly with an appropriate amount of PVP absolute ethanol solution. Soft material, granulated with a 14-mesh sieve, dried at 50-60°C for 1 hour, added the prescribed amount of magnesium stearate and granulated with a 14-mesh sieve. Take the granules and press them into tablets with a special diamond-shaped special-shaped die.
Embodiment 3
[0170] The preparation of embodiment 3 capsules
[0171] G-1 20g
[0172] Starch 200g
[0173] Sodium carboxymethyl starch 12g
[0174] 5% PVP absolute ethanol appropriate amount
[0176]
[0177] Make 1000 capsules
[0178] G-1 and other excipients in the prescription were passed through a 100-mesh sieve, and the prescription amount of G-1 was weighed and mixed with starch and sodium carboxymethyl starch in equal amounts, and the soft material was made with an appropriate amount of PVP absolute ethanol solution, 14 mesh Sieve and granulate, dry at 50-60°C for 1 hour, add the prescribed amount of magnesium stearate and granulate with a 14-mesh sieve. Get the granules and pack into capsules to make G-1 capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com