1,8-naphthalimide derivative as well as synthesis method and application thereof
A synthesis method and product technology, applied in the field of medicine, can solve the problems that have not been seen before, and achieve good medicinal value and remarkable anti-tumor activity in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1) Add 2.77g of 4-bromo-1,8-naphthalene dicarboxylic anhydride (10mmol) into 150ml of ethanol, stir to form a suspension, then add 1.65g of 3,4-methylenedioxyphenethylamine (10mmol ) was added to the suspension, heated to reflux for 8 hours. After the reaction was completed, it was cooled, filtered, and the filter cake was recrystallized with dichloromethane to obtain 3.81 g of an intermediate product with a yield of 90%.
[0030] 2) Dissolve 4.23g of the intermediate product (10mmol) in 50mL of DMSO, add 1.02g of N-dimethyl-1,3-diaminopropane (10mmol), and heat to 135°C for 8 hours. After the reaction was completed, the reactant was put into ice water, and the yellow solid was filtered out, which was the crude product.
[0031] 3) The crude product was purified by silica gel column chromatography (elution solvent: dichloromethane / methanol=1:20, volume ratio) to obtain 2.23 g of a yellow solid product with a yield of 50%.
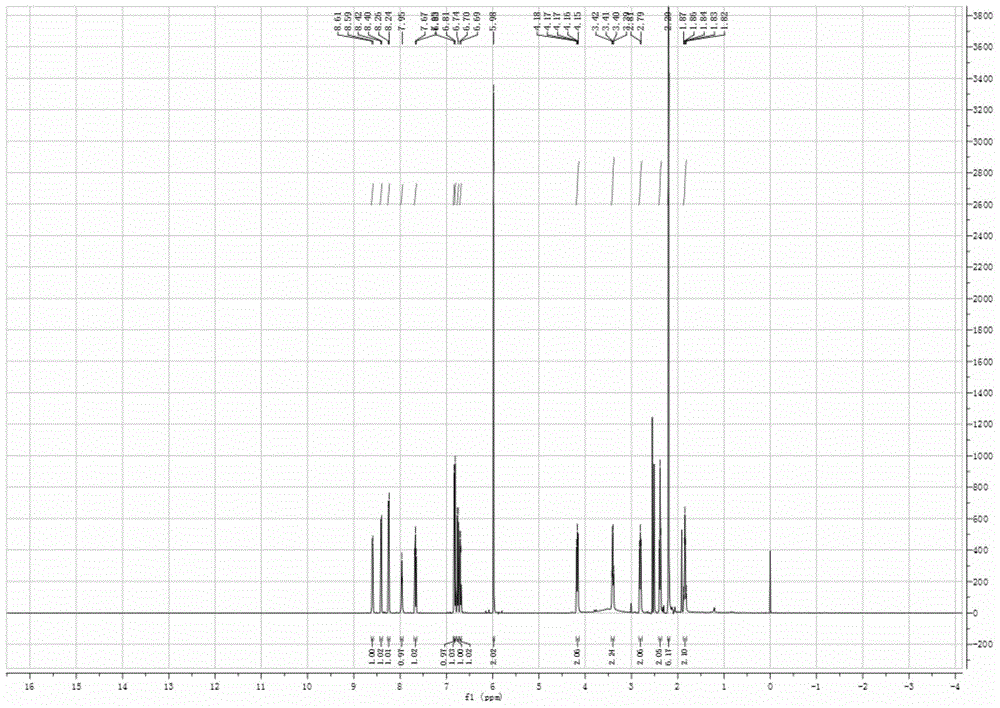
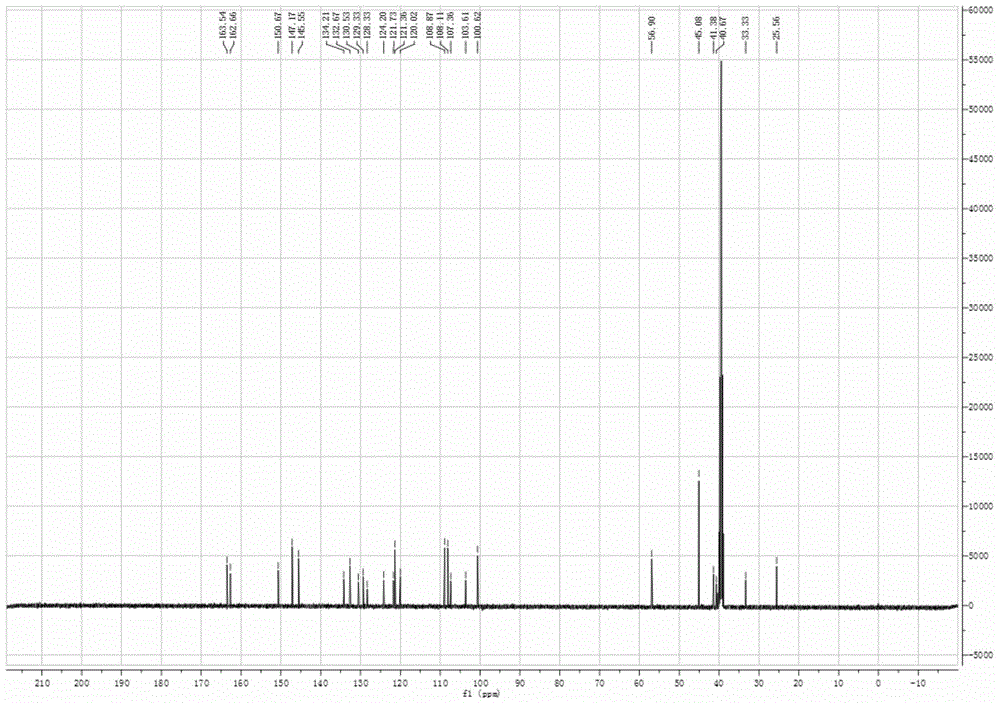
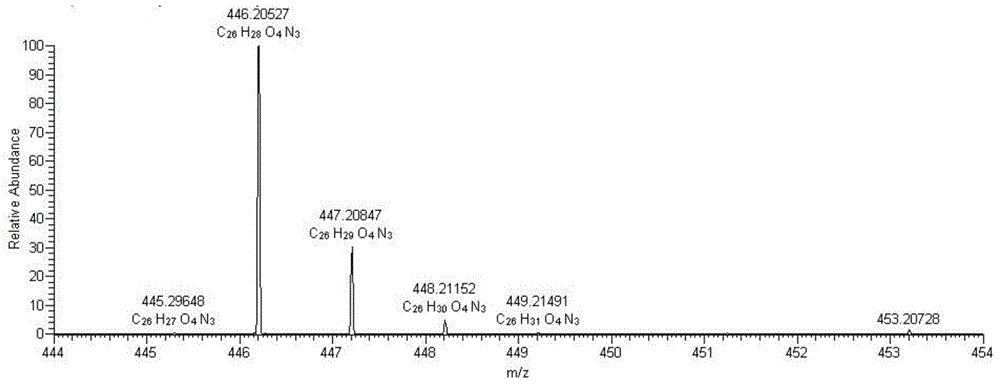
[0032] The resulting yellow solid product is...
Embodiment 2
[0040] 1) Add 2.77g of 4-bromo-1,8-naphthalic anhydride (10mmol) into 150ml of methanol, stir to form a suspension, then add 1.65g of 3,4-methylenedioxyphenethylamine (10mmol ) was added to the suspension, heated to reflux for 8 hours. After the reaction was completed, it was cooled and filtered, and the filter cake was recrystallized twice with dichloromethane to obtain 3.0 g of an intermediate product with a yield of 71%.
[0041] 2) Dissolve 4.23g of the intermediate product (10mmol) in 50mL of DMSO, add 1.02g of N-dimethyl-1,3-diaminopropane (10mmol), and heat to 150°C for 8 hours. After the reaction was completed, the reactant was put into ice water, and the yellow solid was filtered out, which was the crude product.
[0042] 3) Purify the crude product by silica gel column chromatography (elution solvent is dichloromethane / methanol=1:50, volume ratio) to obtain N-(3,4-methylenedioxyphenethyl)-4-(3- N,N-dimethylamino)propylamino-1,8-naphthalimide 2.14g, yield 48%.
Embodiment 3
[0044] 1) Add 2.77g of 4-bromo-1,8-naphthalene dicarboxylic anhydride (10mmol) into 150ml of ethanol, stir to form a suspension, then add 1.65g of 3,4-methylenedioxyphenethylamine (10mmol ) was added to the suspension, heated to reflux for 10 hours. After the reaction was completed, it was cooled and filtered, and the filter cake was recrystallized with chloroform to obtain 3.81 g of an intermediate product with a yield of 90%.
[0045] 2) Dissolve 4.23g of the intermediate product (10mmol) in 50ml of ethylene glycol methyl ether, add 1.02g of N-dimethyl-1,3-diaminopropane (10mmol), and heat to 110°C for 8 hours. After the reaction was completed, the reactant was put into ice water, and the yellow solid was filtered out, which was the crude product.
[0046] 3) Purify the crude product by silica gel column chromatography (elution solvent is dichloromethane / methanol=1:100, volume ratio) to obtain N-(3,4-methylenedioxyphenethyl)-4-(3- N,N-dimethylamino)propylamino-1,8-naphthal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com