Preparation method of monoterpenoid indole alkaloid compound and application thereof
A technology of indole alkaloids and compounds, which can be used in drug combination, organic chemistry, antineoplastic drugs, etc., and can solve the problem of rare rose trees
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
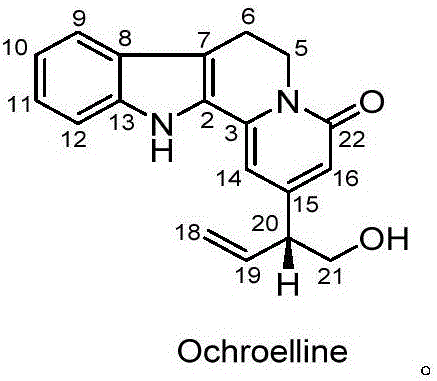
[0018] Embodiment one: the preparation method of compound ochroelline (one)
[0019] 1. After the pulverized rose branches and leaves dried in the shade (28.2kg, Guangdong) were extracted by cold soaking with 3 times the concentration of 90% ethanol solution for 3 times, each extraction was performed for one week, filtered, and the extract was concentrated under reduced pressure to obtain 1286.3g of ethanol extract;
[0020] 2. Add water to the ethanol extract to make a suspension, extract with an equal volume of petroleum ether and an equal volume of ethyl acetate successively, each extracting 3 times, to obtain a petroleum ether extract; the petroleum ether extract is concentrated under reduced pressure, Obtain petroleum ether extract 280.8g;
[0021] 3. Purify the petroleum ether extract by column chromatography: the petroleum ether extract is separated by silica gel column chromatography, and petroleum ether-acetone gradient elution (95:5, 90:10, 80:20, 70:30, 60 :40, 50:...
Embodiment 2
[0026] Embodiment two: the preparation method of compound ochroelline (two)
[0027] 1. After the pulverized rose branches and leaves dried in the shade (9.8kg, Hainan) were extracted by cold soaking with 4 times the amount of methanol for 4 times, each time for 3 days, filtered, the extract was concentrated under reduced pressure to obtain a methanol extract (528.2g).
[0028] 2. Add water to the methanol extract to make a suspension, sequentially extract with equal volumes of petroleum ether and equal volumes of ethyl acetate, and extract 4 times each to obtain petroleum ether extracts; concentrate the petroleum ether extracts under reduced pressure to obtain petroleum ether extracts. ether extract (102.6 g);
[0029] 3. Separation and purification of the petroleum ether extract by column chromatography: segment the petroleum ether extract by silica gel column chromatography, and gradient elution of petroleum ether-acetone (95:5, 90:10, 80:20, 70:30, 60:40, 50:50), collect ...
Embodiment 3
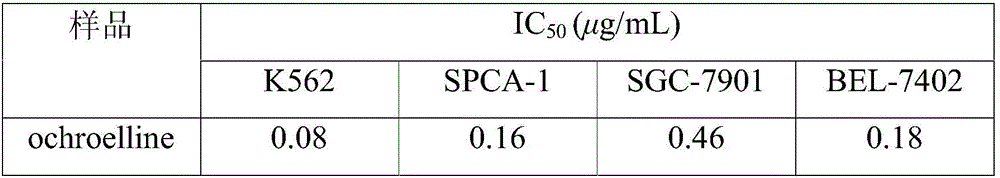
[0031] Example 3: Research on the antitumor activity of the compound ochroelline
[0032] 1. Experimental method: Four common tumor cell lines K562, SPCA-1, SGC-7901 and BEL-7402 were respectively used in RPMI-1640 medium containing 10% calf serum at 37°C and 5% CO 2 cultured in an incubator. The MTT method was used to carry out the cell proliferation inhibition test. The main operation was: take the tumor cell line in the logarithmic growth phase, digest it with 0.25% trypsin, and prepare 5×10 RPMI-1640 culture solution with 10% newborn calf serum 4 cells / mL of cell suspension, inoculated in 96-well plate, inoculated 180 μL per well. at 37°C, 5% CO 2 Cultivate for 8-10 hours under saturated humidity conditions, and when they adhere to the wall, add a sample solution prepared with PBS to each well so that the final concentrations of the samples are 0.1, 1, and 10 μg / mL, respectively. For each concentration, 3 wells were paralleled, and after 44 hours of continuous culture, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com