Acetylpyrazine thiosemicarbazone metal chelating agent and preparation and application of metal complex of the acetylpyrazine thiosemicarbazone metal chelating agent
A technology of acetylpyrazine amino and diethyl semi-thiourea, which is applied in the field of medicine, can solve the problems that other metal-based compounds are useless and make significant progress, and achieve significant in vitro antitumor activity and good medicinal use. The effect of value, principle science
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Synthesis of acetylpyrazine thiosemicarbazone metal chelating agent and its metal complexes.
[0028] The synthetic route is as follows:
[0029]
[0030] Concrete synthesis method is:
[0031] 1) Dissolve 1 mmol of 2-acetylpyrazine (0.122 g) in 20 ml of ethanol (concentration of solvent ethanol is 50 v / v%), stir at 80 °C for 15 min to obtain a solution, and dissolve the above solution one by one Add 1 mmol 4,4-diethylthiosemicarbazone (0.147 g) dropwise into 20 ml of ethanol (solvent ethanol concentration: 50 v / v%) solution, add 3 drops of concentrated hydrochloric acid, After reflux and stirring at 80°C for 24 hours, a light yellow precipitate was obtained. After filtering the light yellow precipitate obtained above, it was washed three times with absolute ethanol and ether, and after drying, the ligand 2-acetylpyrazine 4,4 - Diethylthiosemicarbazone hydrochloride. Light yellow crystals were obtained after recrystallization from ethanol;
[0032] The...
Embodiment 2
[0041] Example 2: Synthesis of acetylpyrazine thiosemicarbazone metal chelating agent and its metal complexes.
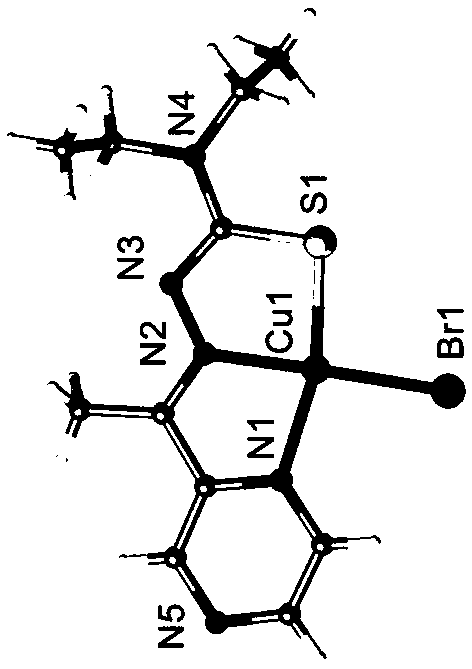
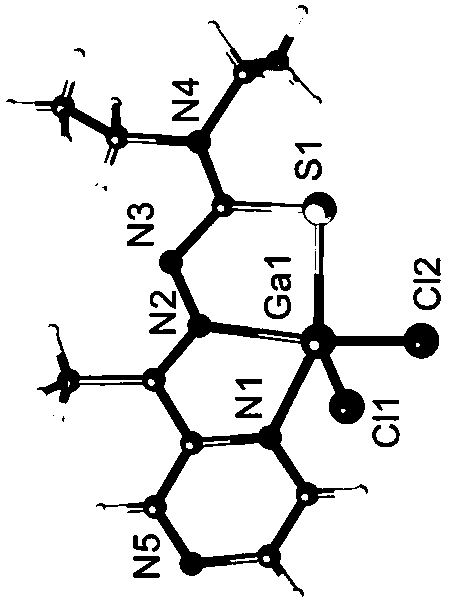
[0042] 2) Add 1 mmol of copper bromide (0.220g), gallium trichloride (0.174g) or ferric chloride (0.161g) in 20ml of methanol (concentration of solvent methanol is 60 v / v%) solution, drop Add 1 mmol of 2-acetylpyrazine 4,4-diethylthiosemicarbazone ligand in 20ml of ethanol (the concentration of solvent ethanol is 50 v / v%) solution, reflux and stir at 20 °C After 2 hours, filter the reacted solution into a 50 ml beaker, seal it with plastic wrap, prick 20 holes and volatilize at 4°C for several days to obtain crystals.
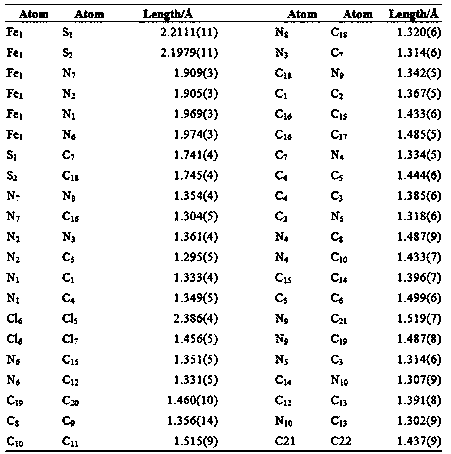
[0043] The obtained crystal is subjected to element analysis, mass spectrometry analysis and X-ray single crystal diffraction analysis, and the specific spectral characteristics are as follows:
[0044] Copper complexes:
[0045] (1) Elemental analysis:
[0046] Chemical formula: C 11 h 16 BrCuN 5 S. Elemental analysis results are: Calculated:...
experiment example 3
[0090] Experimental example 3: The antitumor activity experiment of the acetylpyrazine thiosemicarbazone metal chelating agent and its metal complex (prepared according to the method described in Example 1) and ligands of the present invention.
[0091] 1) Cell lines and cell culture
[0092] Tumor cell lines NCI-H460, A549, HepG2 and HeLa tumor cell lines were all from the American TypeCulture Collection ((Rockville MD, USA), and the cells were placed in an incubator with a volume concentration of 5% at 37 °C with 10% fetal bovine Serum and 100 μgmL -1 Streptomycin, 100 units per ml of penicillin in RPM-1640 medium culture.
[0093] 2) Cell growth inhibition assay (MTT method)
[0094] Note: The full name of MTT is 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazoliumbromide, and the Chinese chemical name is 3-(4,5-dimethylthiazolyl-2)- 2,5-Diphenyl tetrazolium bromide, trade name: thiazolium blue, is a yellow dye.
[0095] After dissolving the acetylpyrazine thiosemi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com