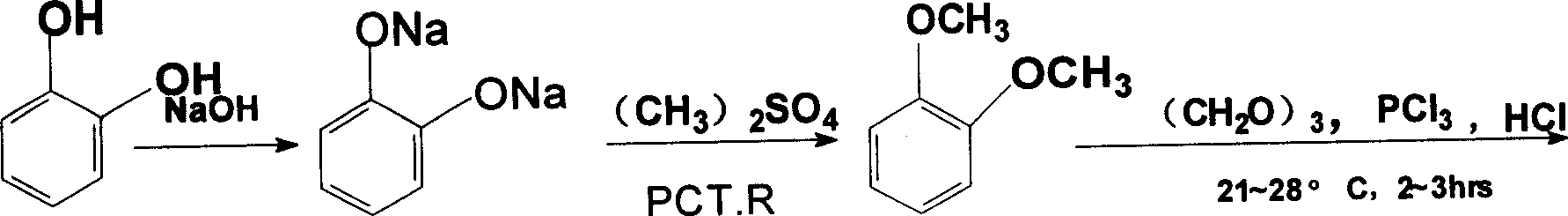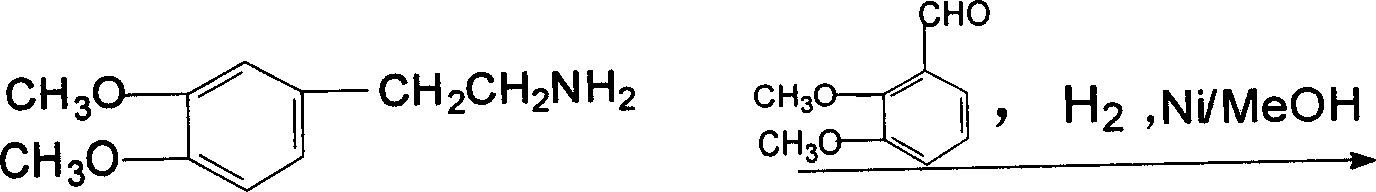Synthesis process of palmatine and its salts
A synthesis method and reaction technology, applied in the field of artificial synthesis technology, can solve the problems that are not conducive to the medical benefits of palmetine, social benefits, economic benefits, the contradiction between resources and medical medication, and the lack of research on the artificial synthesis of palmetine, etc., to achieve The effect of reduced impurity content, low production cost, and stable and reliable products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Palmatine was synthesized according to the following steps:
[0039] a. Etherification: Add 170g of catechol (97.1% content, 1.5mol) to 500mL of water and 126g (3.15mol) of sodium hydroxide into a 1000mL dropping funnel in turn, and stir to completely dissolve the catechol to obtain sodium catechol solution; add 427mL (4.5mol) of dimethyl sulfate and 12g (0.037mol) of bromotetrabutylamine into a 2000mL autoclave, start stirring, heat up to 110°C, and use a plunger pump to dissolve the above-mentioned catechol The sodium solution was poured into the autoclave at a constant speed for 8 hours to react. After the feeding was completed, the temperature was kept at 110°C for another 2 hours. 169g of refined o-dimethoxybenzene was obtained, the gas layer analysis content was 98.87%, and the yield was 79.72%;
[0040] b. Chloromethylation: Add 31g of o-dimethoxybenzene (99% content, 0.24mol) and 7.7g (0.085mol) of paraformaldehyde into a 250mL three-necked bottle, and stir bet...
Embodiment 2
[0052] Palmatine was synthesized according to the following steps:
[0053] a, etherification: operation is the same as embodiment 1, get catechol 170g, wherein the batching ratio of catechol, sodium hydroxide, dimethyl sulfate and benzalkonium chloride is 1: 2.5: 2.1 by molar ratio: 0.08; Obtain 171g of refined o-dimethoxybenzene, gas layer analysis content 98.1%, yield 79.13%;
[0054] B, ethyl cyanation: operation is the same as in Example 1, get 31g of o-dimethoxybenzene, wherein o-dimethoxybenzene, paraformaldehyde, hydrochloric acid, phosphorus trichloride, sodium cyanide, benzalkonium bromide, The proportioning ratio of sodium hydroxide is 1: 0.5: 3.0: 1.5: 1.8: 0.1: 0.04 by molar ratio; 34.3g of o-dimethoxyphenylacetonitrile is obtained, and the content of o-dimethoxyphenylacetonitrile is 96.5%. 77%.
[0055] C, hydrogenation: operation is the same as embodiment 1, get 125g of o-dimethoxyphenylacetonitrile, wherein o-dimethoxyphenylacetonitrile, liquefied ammonia, me...
Embodiment 3
[0059] Preparation of palmatine hydrochloride:
[0060] Palmatine hydrochloride (that is, palmatine, also known as rhubarb): add 30 grams of palmatine (6) to the reaction bottle, add water to dissolve it, add 2 grams of activated carbon, and decolorize at 80-98 ° C for 27-30 minutes , hot filtration, pour the filtrate into the calculated amount of 10% HCl60ml, stir, cool to below 15°C, place it for more than 4 hours, filter, wash the filter cake with cold water several times, drain it, and dry it below 80°C Mating hydrochloride 28.5g, content 96.8%, weight yield is 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com