Zika virus E antigen and application of Zika virus E antigen in fluorescence immunochromatographic reagent
A fluorescence immunochromatography and Zika virus technology, which is applied in the field of fluorescence immunochromatography in medical immunology, can solve the problems of low preparation and expression of Zika virus E antigen protein, false positive detection results, weak specificity, etc. Achieve the effect of simple result determination, wide detection range and strong detection specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
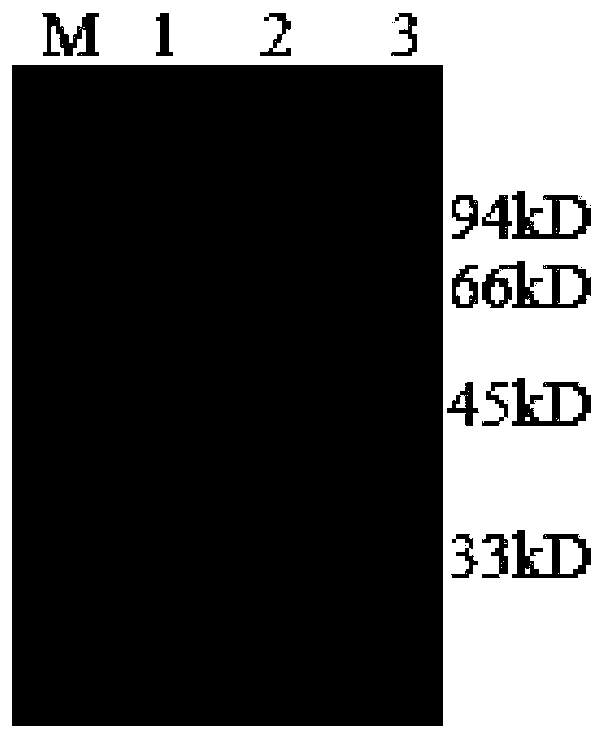
[0033] The preparation of embodiment 1 Zika virus E antigen
[0034] 1. Construction of recombinant plasmids
[0035] Select the Zika virus E protein, its nucleotide sequence (GenBank sequence number: HM133639.1), modify the gene sequence according to the preferred codons of Escherichia coli O127:H6, and analyze the secondary structure of the recombinant protein by bioinformatics After screening, the nucleotide sequence shown in GenBank sequence number: HM133639.1 was ligated into the expression vector PGEX-4T-2 after testing, and the ligated product was obtained as a recombinant plasmid, which was transformed into DH5α competent cells, but the corresponding protein could not be obtained .
[0036] After a large number of experimental verifications, 20 clones were selected for expression, and finally the single clone with the largest expression was selected. The determined gene sequence is shown in SEQ ID NO.1, and the corresponding amino acid sequence is shown in SEQ ID NO.2...
Embodiment 2
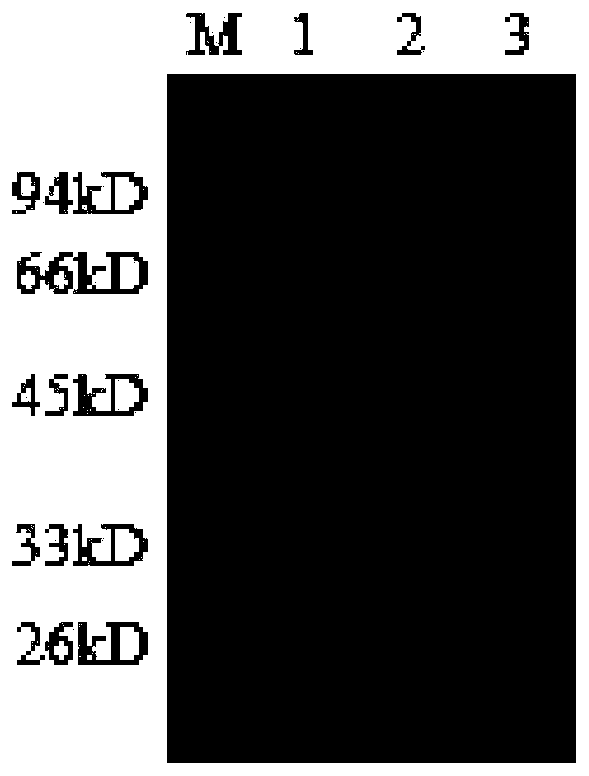
[0041] Example 2 Preparation of Fluorescence Immunochromatography Reagent for Detecting Zika Virus Antibody
[0042] 1. Antibody protein labeled with fluorescent microspheres
[0043] (1) Microsphere activation: 1. Take 200 μL of fluorescent microspheres (bangs carboxyl microspheres), add 1 mL of 0.2 mol / L PBS, and centrifuge at 12000 rpm for 10 minutes. Aspirate and discard the supernatant, repeat centrifugation twice, add 600 μL 0.2mol / LPBS to resuspend for use, take 200 μL 10 mg / mL EDC (N-hydroxysuccinimide) solution and 200 μL 6 mg / mL NHS (N-hydroxysuccinimide) imine) solution. Add the resuspended fluorescent microspheres, vortex and mix well, and place on a rotating reaction rack to react in the dark for 30 minutes.
[0044] (2) Preparation of activated fluorescent microspheres labeled mouse anti-human IgG monoclonal antibody: the activated fluorescent microspheres obtained by the above preparation method were centrifuged at 12000g for 5min, and the supernatant was disc...
Embodiment 3
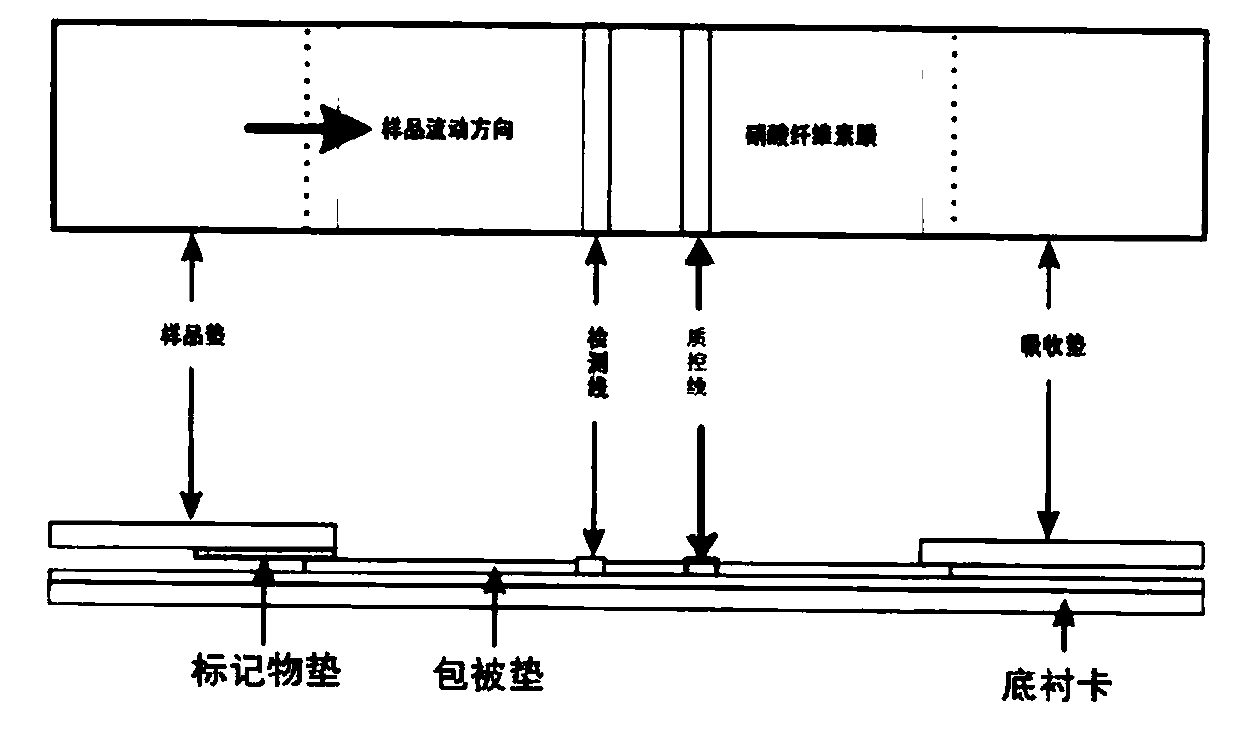
[0057] The detection of embodiment 3 fluorescence immunochromatography reagent sensitivity
[0058] The reagents prepared in Example 2 were used to detect Zika IgG positive samples (provided by Changchun Customs Inspection and Quarantine Center) respectively, and diluted with diluent PBS to 1:10, 1:100, 1:1000, 1:10000, 1:100000, 1:1000000, at the same time, use negative serum as a negative control, take 80 μL of serum samples and add sample pads, and judge the results within 8 minutes. The test result is a positive sample with a detection sensitivity of 1:100000 dilution.
[0059] The E antigen prepared in Example 1 was used to prepare colloidal gold immunochromatography test paper, prepared by conventional methods, using SPA to mark colloidal gold particles, the detection zone on the nitrocellulose membrane was coated with NS1 antigen protein, and the quality control zone Coated goat anti-mouse IgG; the obtained colloidal gold immunochromatographic test paper was diluted to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com