Application of interleukin 21 (IL-21) in preparation of anti-hepatitis B virus (HBV) medicine preparations
A hepatitis B virus, IL-21 technology, applied in the fields of genetic engineering and biomedicine, can solve the problems of large adverse reactions, inability to clear cccDNA of liver cell nucleus, and low patient response rate, so as to improve the conversion rate and reduce hepatitis B. Viral DNA load, effect of durable protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
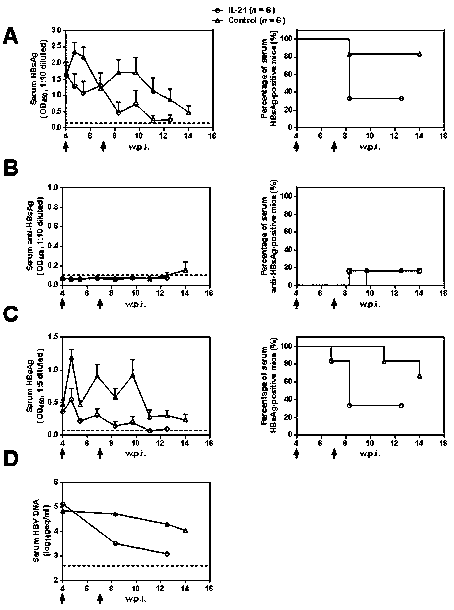
[0031] Example 1: Anti-HBV Intervention Therapy Experiment of IL-21 in Mouse Model
[0032] 10 μg of HBV expression plasmid was dissolved in 2ml of PBS buffer, and injected into 40 male BALB / c mice of 6-8 weeks by tail vein high pressure hydrodynamic method, and 0.2ml of blood was collected through the orbit at 4 w.p.i. The whole blood was treated with anticoagulant, and the separated serum was used for the detection of HBsAg, anti-HBsAg, HBeAg and HBV DNA respectively, among which N mice were all positive for antigen and nucleic acid and negative for antibody in the above three indicators, randomly selected Twelve mice were divided into two groups: IL-21 treatment group (n=6) and control group (n=6), and 25 μg IL-21 was injected by tail vein high pressure hydrodynamic method at two time points of 4 w.p.i and 7 w.p.i. For the overexpression plasmid or the control plasmid (Control), 0.2 ml of blood was collected through the orbit at 8 time points (4.7, 5.4, 6.8, 8.3, 9.7, 11...
Embodiment 2
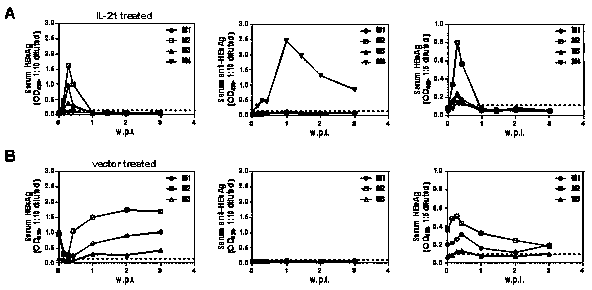
[0038] Example 2: Mice negative for viral antigens after IL-21 treatment acquire durable protection against HBV
[0039] From the IL-21 intervention group in Example 1, randomly select mice (n=4) that were negative for antigens (HBsAg and HBeAg) after the end of the observation period (> four weeks), and randomly select mice in the control group (n=3) , 10 μg of HBV expression plasmid BPS was dissolved in 2ml of PBS buffer, and injected into the experimental group and the control group mice by tail vein high pressure hydrodynamic method, respectively, at six time points (1 day, 2 days, 3 days, 1 w.p.i, 2 w.p.i and 3 w.p.i) 0.2ml of blood was collected through the orbit. The whole blood was treated with anticoagulant, and the separated serum was used for the detection of HBV-related indicators. The serum separated at the above six time points was used for the detection of HBsAg, Anti-HBsAg and HBeAg, and each serum was tested separately;
[0040] The results are shown in th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com