Lignan compound and preparation method thereof
A technology of lignans and compounds, applied in the direction of active ingredients of heterocyclic compounds, organic chemistry, antiviral agents, etc., can solve the problems of insufficient disclosure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
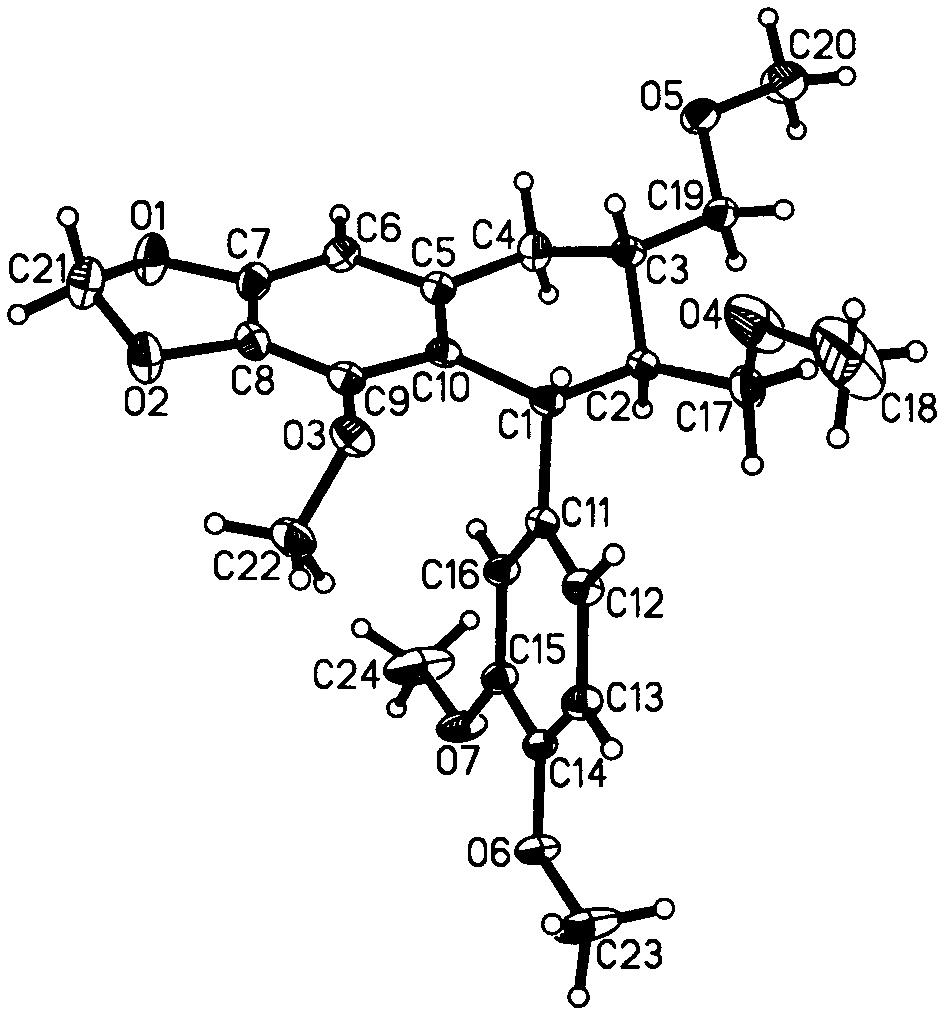
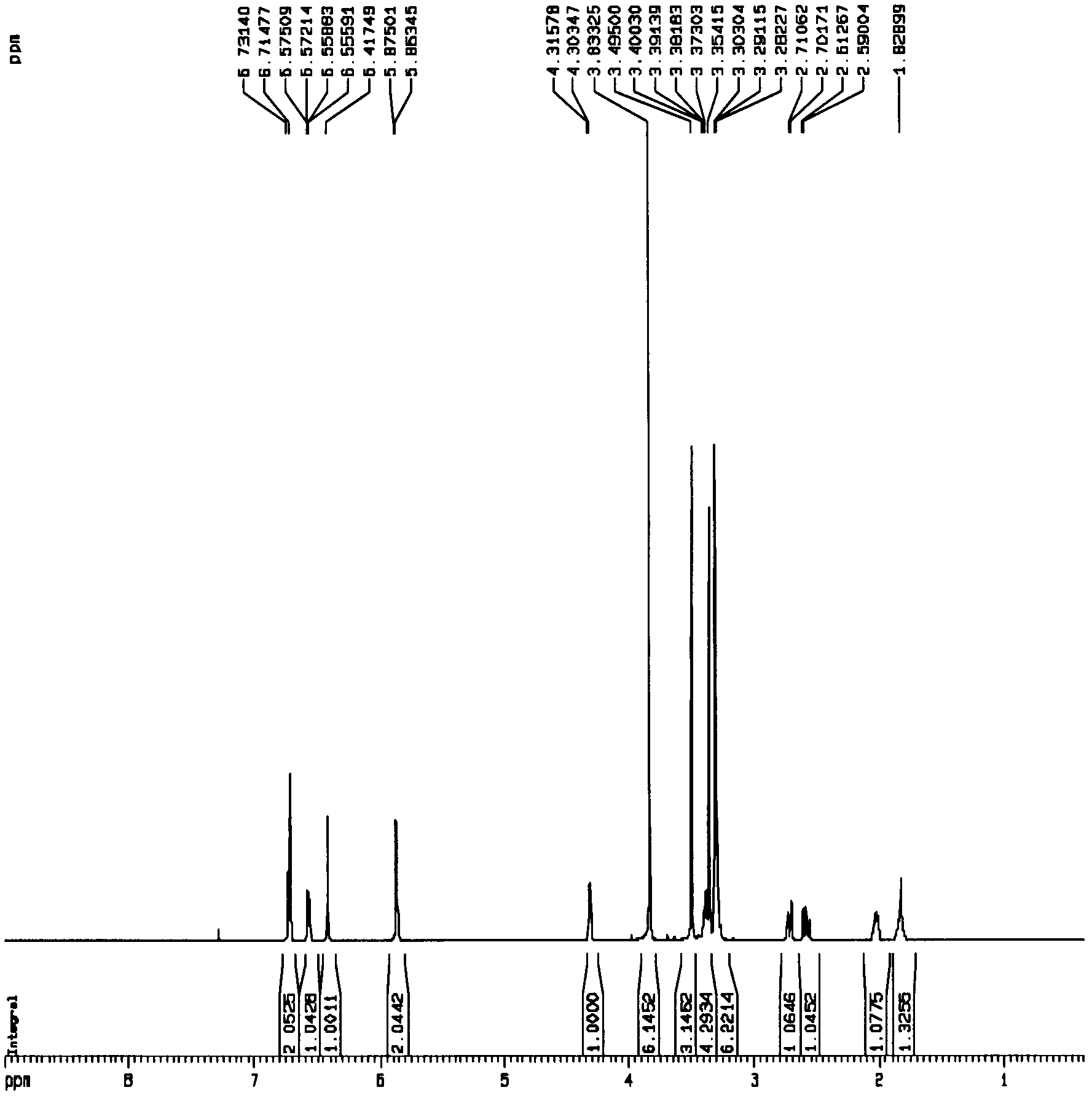
[0049] Get 20.0 kilograms of shady and dry bead grass (Phyllanthus Niruri Linn.), crush it to 5-10 mm fragments, pour it into an extraction tank (equipped with a solution circulation pump) with a capacity of 200 liters, add 100 liters of 95% ethanol, Soak at room temperature (20-30°C) for 2 days (during which the circulation pump runs to circulate the solution). The solution was then separated. The extraction was repeated 4 times with solvent. The solution is first filtered through a strainer. The solution obtained by filtration was concentrated under reduced pressure (concentration temperature ≤ 45°C. 1.8 kg of (5R, 6S, 7R)-5-(3',4'-dimethoxyphenyl)-4-methoxy-6 , 7-bis(methoxymethyl)-5,6,7,8-tetrahydronaphtho[2,3-d][1″,3″]dioxole (namely neolignan Compounds) extract A. (5R, 6S, 7R)-5-(3',4'-dimethoxyphenyl)-4-methoxy-6,7-di(methoxymethyl) - The extraction rate of 5,6,7,8-tetrahydronaphtho[2,3-d][1″,3″]dioxole was 85.0%.
Embodiment 2
[0051] Get 20.0 kg of cool and dry bead grass (Phyllanthus Niruri Linn.), crush it to 5-10 mm fragments, pour it into a 200-liter extraction tank (heatable, without stirring device, with reflux condensing device), add Into 100 liters of 95% ethanol, heated to reflux temperature (78-80° C.), and maintained for 3 hours. The solution separated after cooling. The extraction was repeated 4 times with solvent. The solution is first filtered through a strainer. The solution obtained by filtration was concentrated under reduced pressure (concentration temperature≤45°C. 2.1 kg of extract A was obtained. (5R, 6S, 7R)-5-(3',4'-dimethoxyphenyl)-4-methoxy Extraction rate of base-6,7-di(methoxymethyl)-5,6,7,8-tetrahydronaphtho[2,3-d][1″,3″]dioxole 95%.
Embodiment 3
[0053] 2 kg of extract A was dissolved in 5 liters of 95% ethanol, and saturated ammonium sulfate aqueous solution was added under constant stirring until two immiscible items appeared, and the volumes of the two were equal. Then extract with the solvent petroleum ether (boiling range 60-90° C.) equal to the volume of the upper phase to obtain petroleum ether extract. After the extract was concentrated, 450 grams of sherwood oil part extract B was obtained.
[0054] 450 grams of petroleum ether fraction extract B was dissolved in 2 liters of 95% ethanol in heat (45-50° C.), and then crystallized by cooling. A solid material and a solution were separated. Add 1.6 liters of water to the solution (the equivalent water content is about 40% of the solution volume), and then cool (2-4°C) to precipitate. Concentrate to obtain 110 grams of extract C after separating the solution. This 60 grams of extract C was dissolved in ethyl acetate to form a solution with a mass percent concen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com