Application of luteolin 7-O-glucoside in preparing anti-HBV drug
A technology of luteolin and glucoside, which is applied in antiviral agents, pharmaceutical formulas, medical preparations containing active ingredients, etc., and can solve problems such as no reports of anti-hepatitis B virus activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
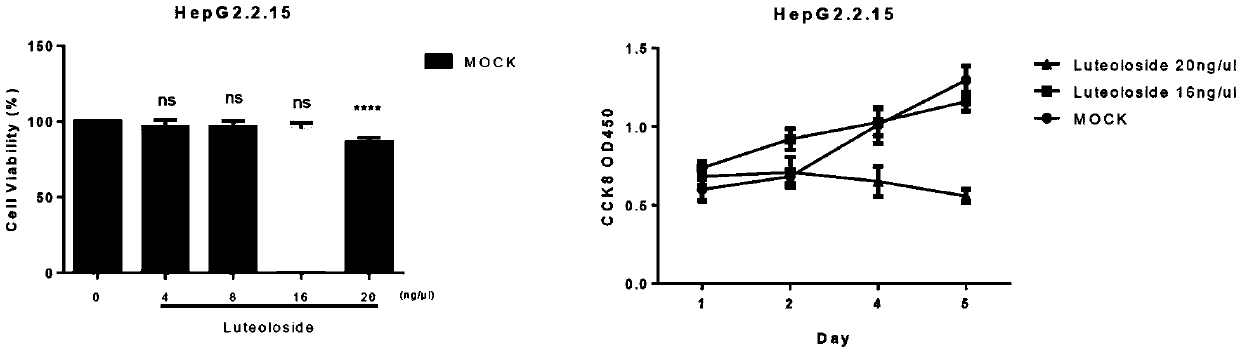
[0020] Example 1: Compound Luteolin-7-O-glucoside on cytotoxicity and proliferation inhibition experiments
[0021] Determination of the toxicity of the compound luteolin-7-O-glucoside to HepG2.2.15 cells: HepG2.2.15 cells digested with trypsin were inoculated into 96-well plate cells at a density of 5000 cells / well, at 37°C, 5% CO 2 After culturing for 24 hours, discard the liquid, add 100 μl DMEM medium again, and dilute the drug into 4 serial concentrations (respectively: 4 μg / ml, 8 μg / ml, 16 μg / ml, 20 μg / ml), and each dilution has 5 pairs wells, and a normal cell control group was set at the same time. After 48 hours of drug action, the cytotoxicity was detected, and the medium of the same compound concentration was changed once at the same time. After 3 days (the total action time was 5 days), the inhibitory efficiency of the compound on cell growth was detected. The results showed that the compound had no effect on HepG2.2.15 cells. The maximum non-toxic concentration c...
Embodiment 2
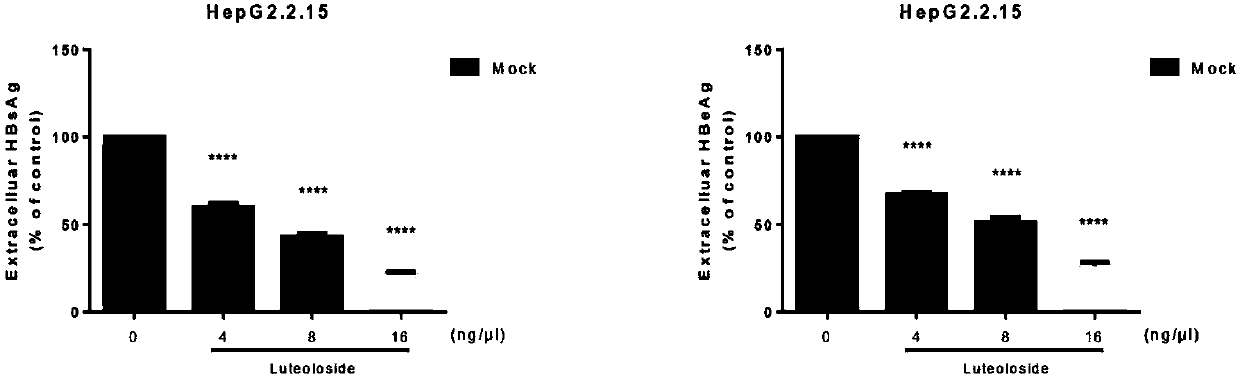
[0022] Example 2: Anti-hepatitis B virus effect experiment of compound luteolin-7-O-glucoside
[0023] Determination of the inhibitory effect of the compound luteolin-7-O-glucoside on the hepatitis B virus antigen secreted by HepG2.2.15 cells: ELISA kits were used to detect the levels of hepatitis B virus surface antigen and e antigen in the supernatant collected from the cells, and the operation Proceed according to the instructions of the kit, and finally read the OD value at a wavelength of 450nm with a microplate reader, and compare the inhibitory effect of the reaction compound on the intracellular antigen according to the percentage of the control group;
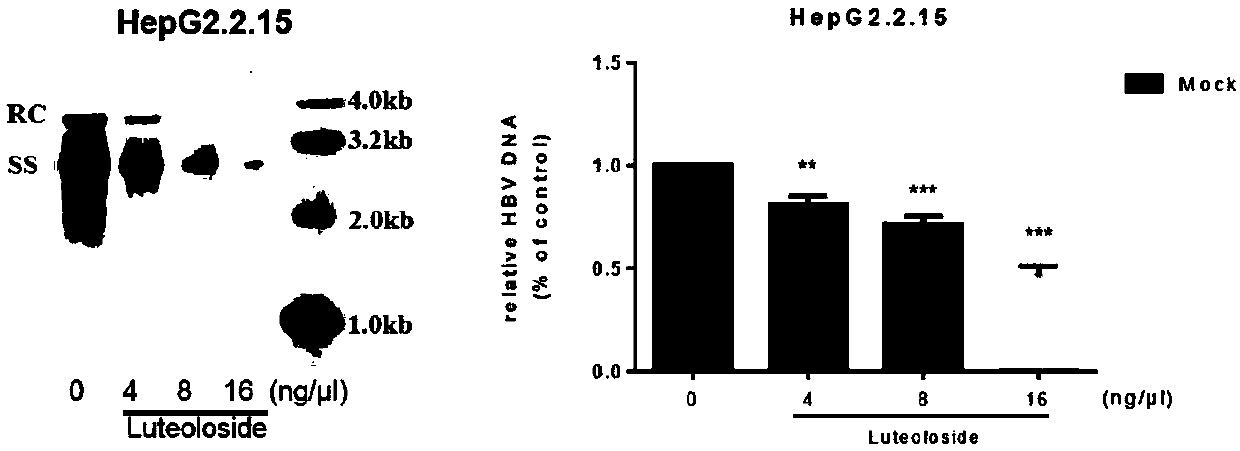
[0024] Determination of the inhibitory effect of the compound luteolin-7-O-glucoside on the replication of hepatitis B virus particles in HepG2.2.15 cells: after the compound acts on HepG2.2.15 cells for 72 hours, the cells are harvested and broken, digested with DNase, protease After K digestion, phenol-chloroform ext...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com