Methods of inducing cytotoxic immune response and recombinant simian ademovirus compositions useful therein
A technology of simian adenovirus and recombinant adenovirus, applied in the direction of complete cells/viruses/DNA/RNA components, medical materials derived from viruses/bacteriophages, viruses, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] C. Preparation of Recombinant Virus Particles
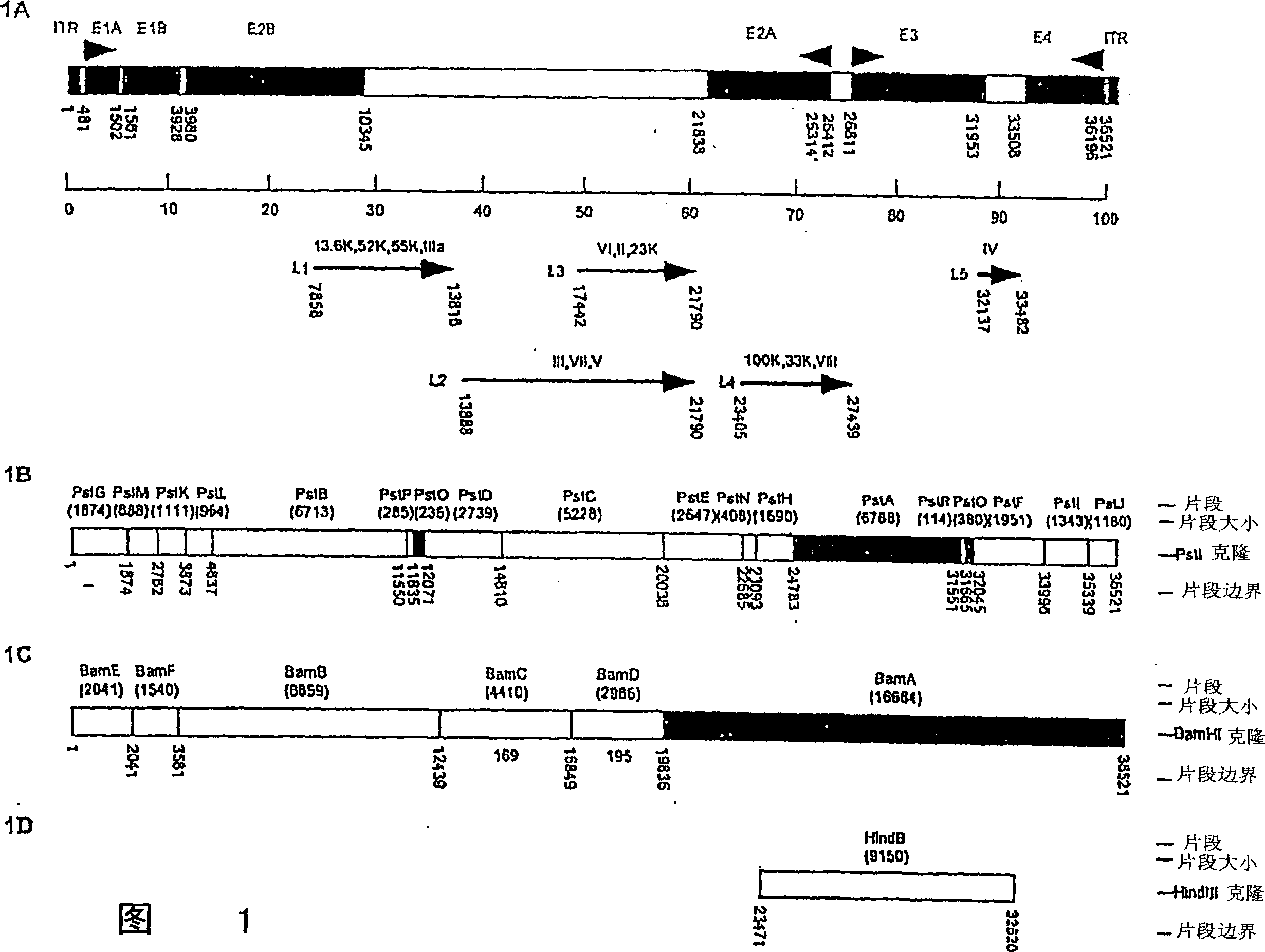
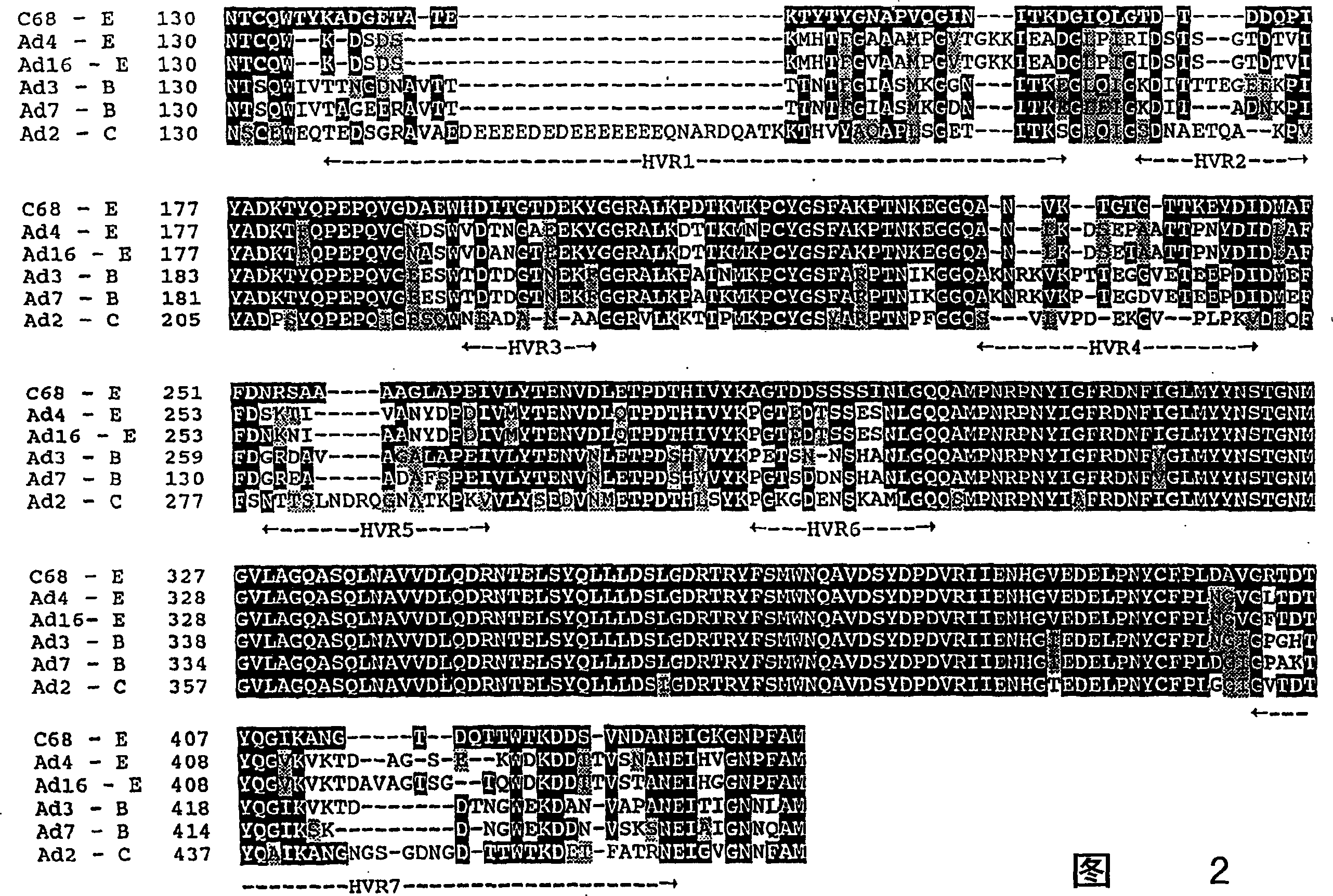
[0031] Suitable recombinant simian adenoviruses are prepared using methods well known to those skilled in the art, such as those described in US Pat. No. 6,083,716. In constructing recombinant simian adenoviruses for delivery of heterologous molecules to a subject (eg, a human, canine, feline, or other mammal), the adenoviral nucleic acid sequences employed in the vector are available from a variety of simian sources.
[0032] Vectors containing simian (eg, chimpanzee) adenoviral sequences lacking the simian adenoviral sequences required for the production of infectious recombinant viral particles can be used in combination with a helper virus or vector. The helper virus provides the gene products required for simian adenovirus infectivity and propagation. When selective deletions of one or more simian adenovirus genes are made in functional viral vectors, these deleted gene products can be provided during viral vector pr...
Embodiment 1
[0070] Example 1 - Preparation of E1 deletion vector based on chimpanzee adenovirus C68
[0071] A replication-defective form of C68 was isolated for gene transfer. A typical strategy to obtain E1-deleted recombinants is by homologous recombination in E1-expressing cell lines. The first step is to generate a plasmid containing m.u.0 to 1.3 followed by the addition of a minimal gene expressing enhanced green fluorescent protein (GFP) under the CMV promoter and the C68 sequence spanning 9-16.7 m.u. The linearized plasmid and the SspI digested C68 plasmid (cut by SspI at 3.6 m.u, leaving 4644 bp for homologous recombination) were co-transfected into a cell line expressing El. Experiments were initially performed with 293 cells carrying human Ad5 E1 and it was hoped that this would be sufficient for trans complementation. In fact, the plaques formed provided the desired recombinants. The resulting vector was named C68-CMV-GFP.
[0072] Strategies to improve recombinant product...
Embodiment 2
[0081] Example 2 - Antigen Expression (Gag Secretion) in TK Cells Infected with Simian Adenovirus Vaccine Constructs
[0082] As described in Example 1 and Z.Q.Xiang, et al., Virol.219, 200 (196), recombinant chimpanzee adenovirus strain 68 (Adchimp68) and human adenovirus strain 5 (Adhu5), both carrying modified HIV - Nucleotide sequence of the truncated gag gene of clade B. The transcripts of HIV-1's structural proteins (including gag) contain genetically unstable elements and require the presence of rev protein in order to export the nucleus and efficiently express in the cytoplasm (S. Schwartz et al., J Virol 66, 7176 (1992); S. Schwartz et al., J Virol 66, 150-159 (1992); G. Nasioulas et al., J Virol 68, 2986 (1994)). Adenoviruses rely on nuclear transcription and thus require rev to express HIV-1 proteins. To avoid dependence on Rev, the genetic instability element in the codon-modified gag sequence was removed by site-directed mutagenesis (R. Schneider et al., J Virol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com