Method for preparing thymosin polypeptide by using interin
A technology of thymosin and intein, which is applied in the field of preparation of thymosin polypeptides, can solve problems such as fragmentation and inhomogeneity of polypeptide products, and achieve the effects of simple process, easy operation and industrialization, and simple reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1. Construction of pET32a / intein / Thymosin α-1 expression plasmid.
[0034] Synthesize thymosin alpha 1 (Thymosin α-1) gene, which contains LguI at the 5' end and XholI restriction site at the 3' end, and clones the synthesized gene into the pMD18-T vector (the above steps are all completed by the gene synthesis company ).
[0035] The resulting pMD18-T Vector / Thymosinα-1 plasmid was transformed into Escherichia coli DH5α competent cells. The transformation method is as follows: take 1 μL plasmid and add it to 100 μL E. coli DH5α competent cells, place on ice for 30 minutes; then place in a water bath at 42°C for 90 seconds, and then return to ice for 3-5 minutes; then add 800 μL LB Culture medium (Luria-Bertani medium), incubate at 37°C for 45min; then centrifuge at 2500rpm for 5min to collect the bacteria, discard 700 μL of medium, and spread the remaining 200 μL of medium on an agarose plate containing 50 μL / mL ampicillin for culture overnight. Pick the single clo...
Embodiment 2
[0046] 1. Construction of pTwin-1 / Thymosin α-1 expression plasmid.
[0047] As described in Example 1, the pMD18-T Vector / Thymosin α-1 plasmid was purchased from a gene synthesis company, transformed into Escherichia coli DH5α competent cells, and then the plasmid was extracted with a plasmid extraction kit, and Thymosin α-1 was obtained by PCR amplification Gene, the obtained target gene fragment is 90bp.
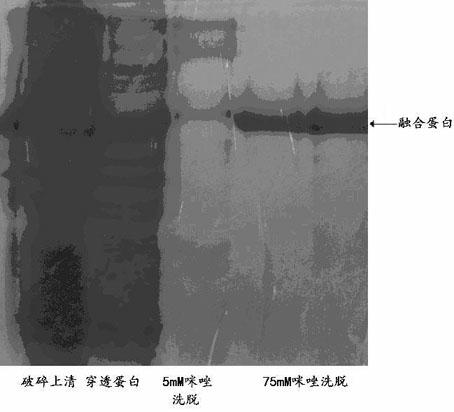
[0048] Digest the pTwin-1 plasmid with LguI and PstI. The reaction system is: 10 μL of pMD18-T Vector / Thymosin α-1 plasmid, 2 μL of digestion buffer, 1 μL of NcoI and XholI enzymes, add water to 20 μL, and incubate at 37°C React for 15 minutes. The double-digested product was purified by agarose gel electrophoresis and recovered with a gel extraction kit. The fragment size was 7375bp.
[0049] Ligate the recovered target gene fragment and the pTwin-1 vector digested with LguI and PstI (treated with CIAP to remove the blunt-end phosphate group) with T4-DNA ligase to for...
Embodiment 3
[0053] Example 3: In vitro acetylation of recombinant thymosin 28 peptide.
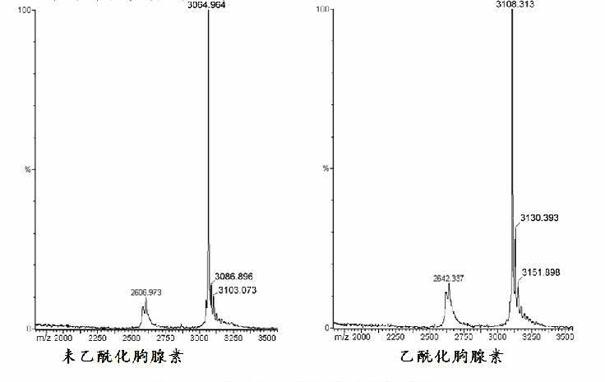
[0054] The molecular weight of thymosin α-1 is 3108Da, and the molecular weight of unacetylated thymosin is 42 smaller than it, which is 3066. See image 3 shown.
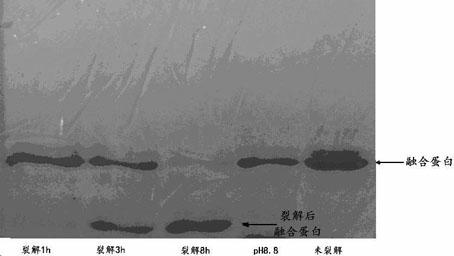
[0055] Use acetic acid to adjust the pH of the thymosin α-1 28 peptide solution obtained in Example 1 or Example 2 to 2-3, then add acetic anhydride at a rate of 2 μL of acetic anhydride per 10 mL of solution every 10 min, and keep on ice Stir the reaction. The reaction was monitored by RP-HPLC, and the reaction was stopped when acetylated thymosin was no longer accumulated. The natural structure thymosin α-1 was purified by RP-HPLC. see Figure 4As shown, Ⅰ, Ⅱ, Ⅲ, and Ⅳ in the figure respectively represent fusion protein cleavage, fusion protein cleavage purified by DEAE, purified thymosin 28 peptide subjected to in vitro acetylation reaction, and RP-HPLC purification after acetylation reaction peaks 1, 2, and 3 represent unacetylate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com