New application of recombinant human thymosin alpha collagens
A technology of thymosin and proprotein, applied in the field of recombinant human thymosin alpha proprotein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] The following embodiments will further illustrate the present invention in conjunction with the accompanying drawings.
[0019] For the recombinant protein monomer and fusion protein described in the present invention, please refer to the applicant's Chinese Patent 200810072084.4 (A Recombinant Protein in the Preparation of Oral Drugs for Preventing Diabetes) and Chinese Patent 200710009083.0 (A Preparation Method for Anti-tumor Fusion Protein) and its applications).
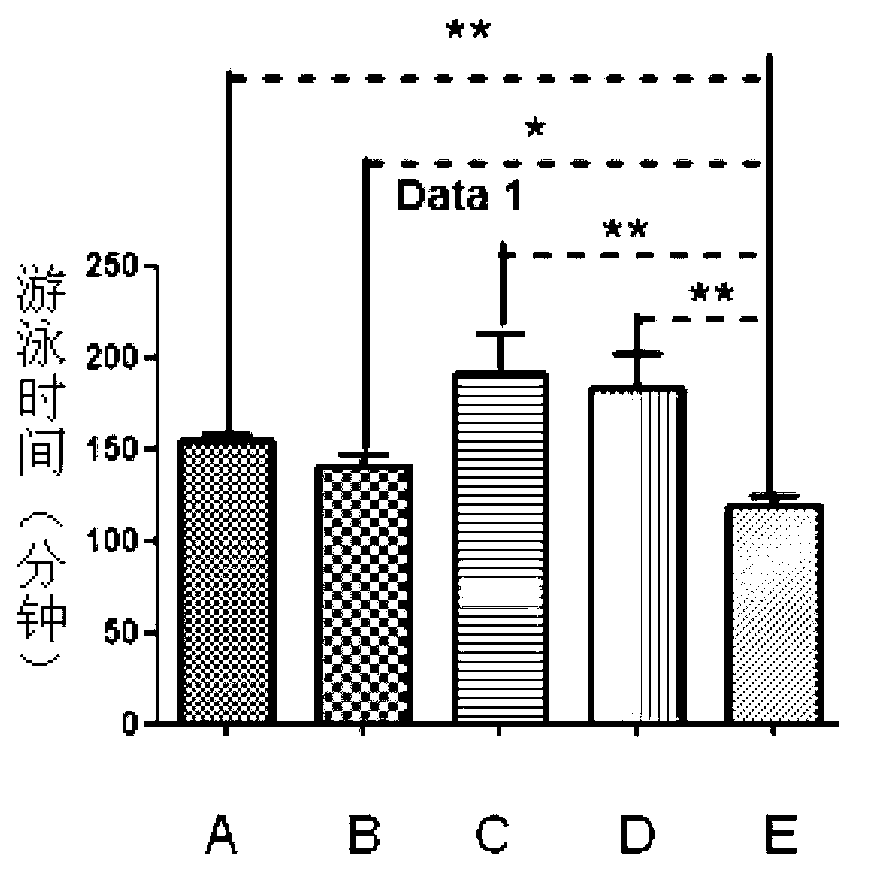
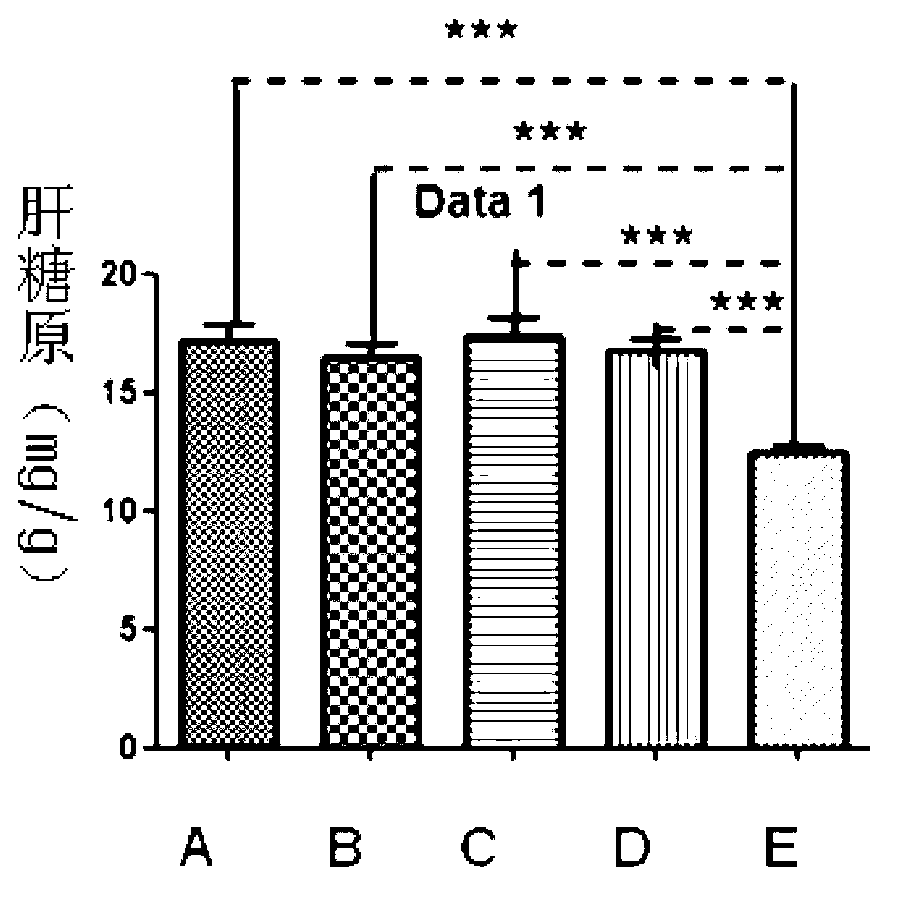
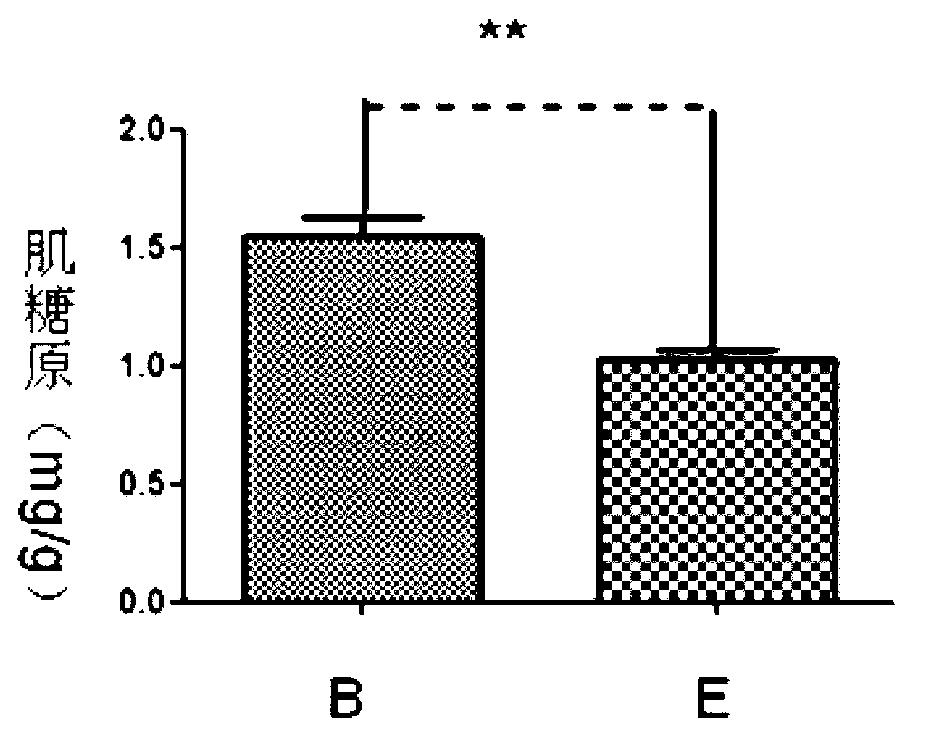
[0020] Anti-fatigue test of prothymosin alpha monomer and GST-prothymosin alpha fusion protein. Specific steps are as follows:
[0021] ①Choose 4-5 weeks old Kunming rats, divided them into 5 groups, group A, B, C, D, E, and group E blank control group, injected the same volume of PBS, and injected 1 μg / d thymus in groups A and B respectively. Prothymosin alpha monomer and GST-prothymosin alpha fusion protein, groups C and D were injected with 10 μg / d dose of prothymosin alpha monomer and GST-prothymosi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com