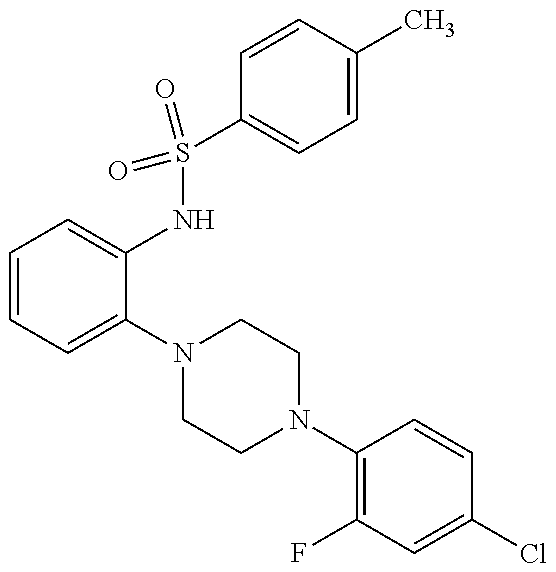
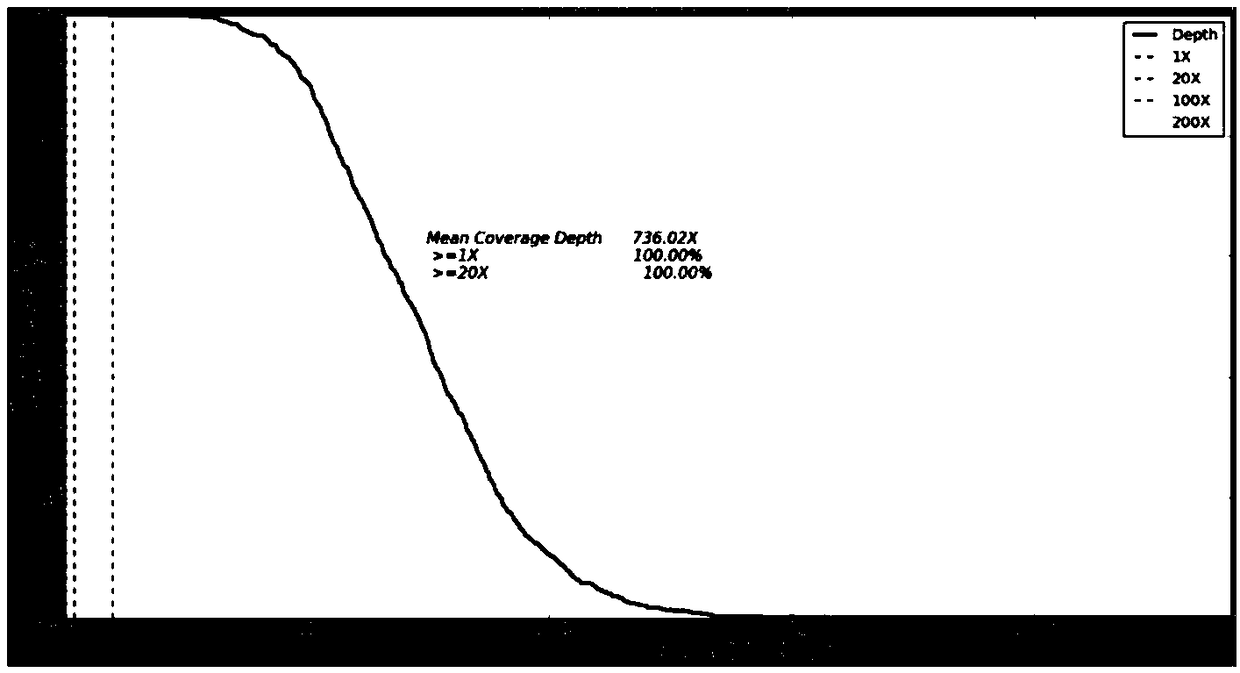
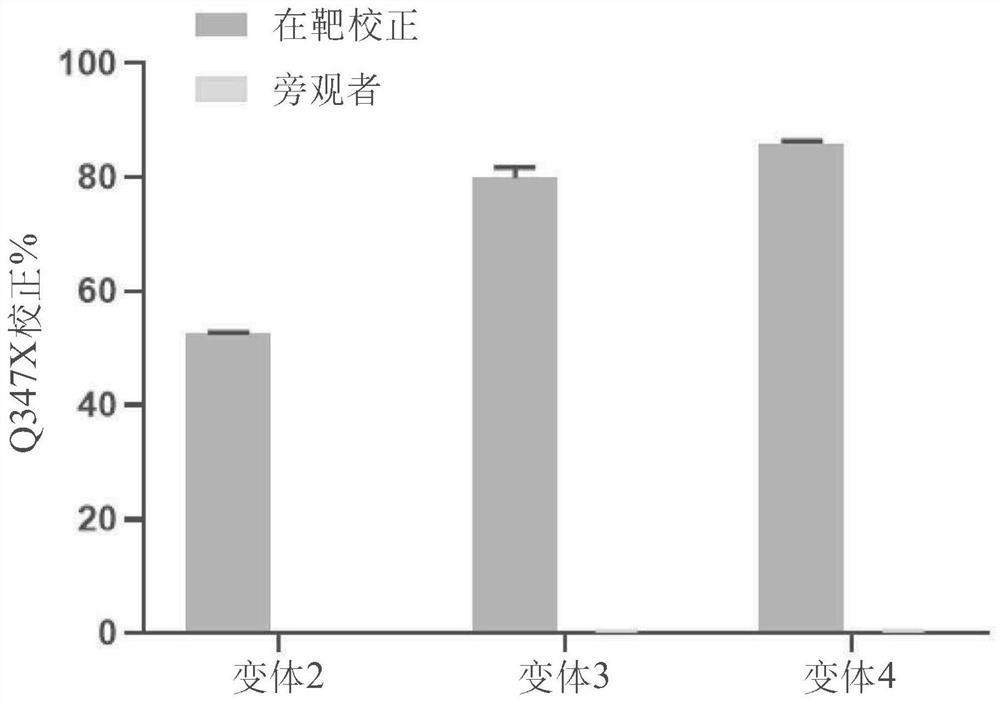
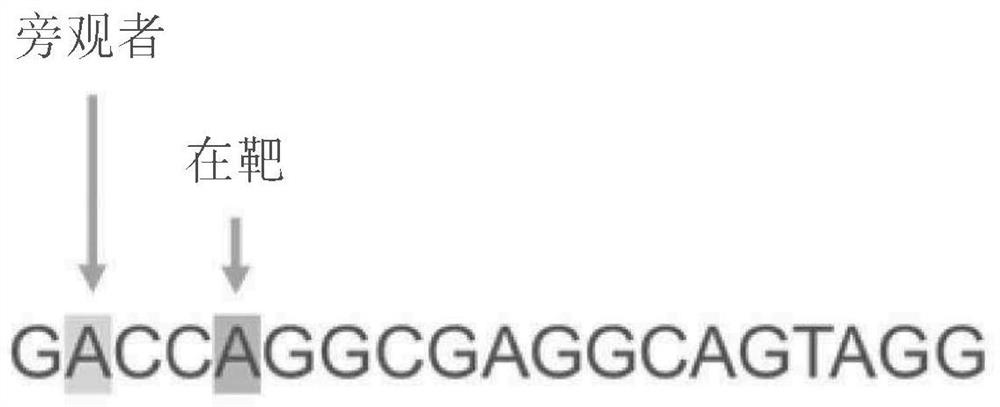Patents
Literature
41 results about "Glycogen storage disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A glycogen storage disease (GSD, also glycogenosis and dextrinosis) is a metabolic disorder caused by enzyme deficiencies affecting either glycogen synthesis, glycogen breakdown or glycolysis (glucose breakdown), typically within muscles and/or liver cells.
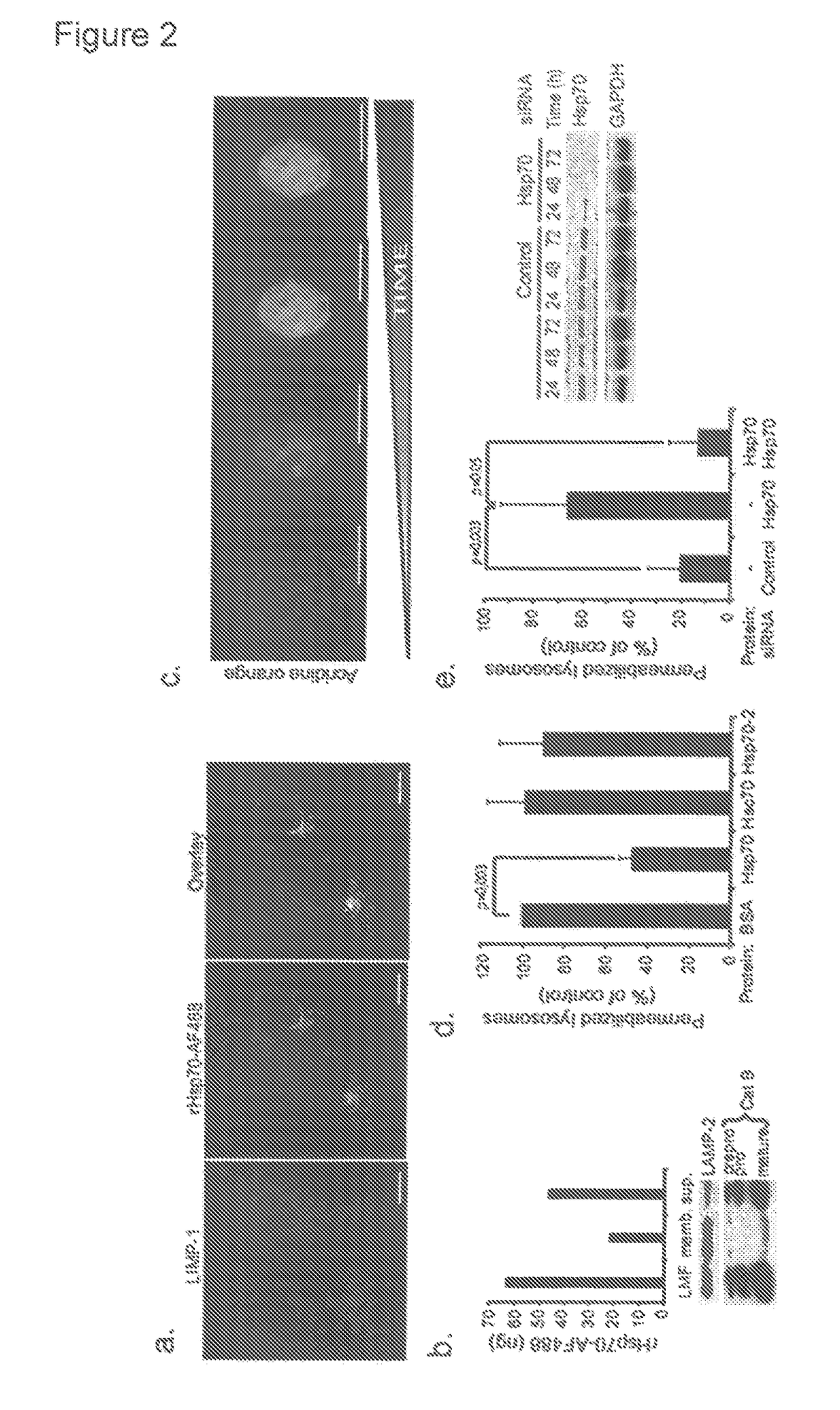
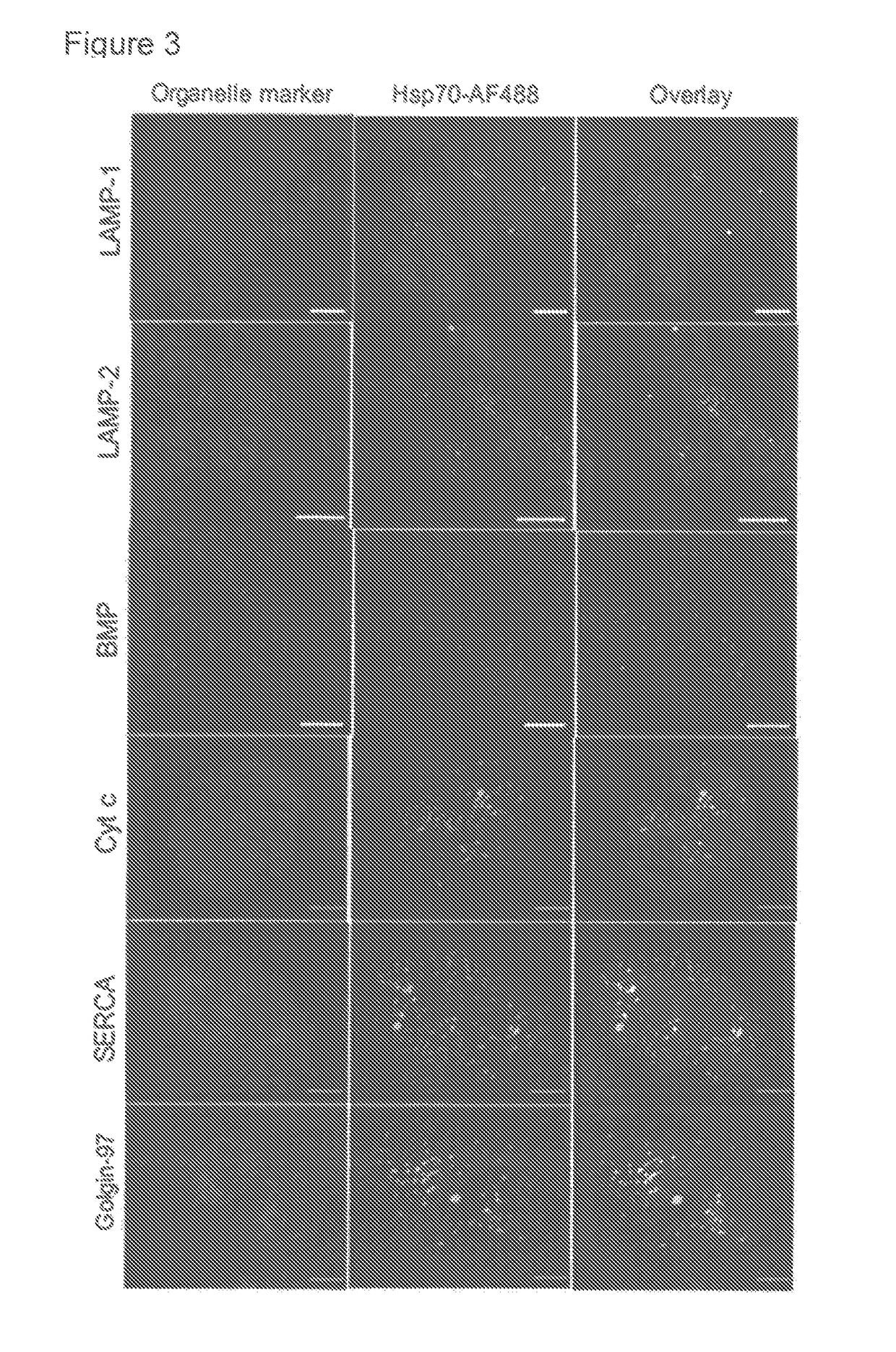
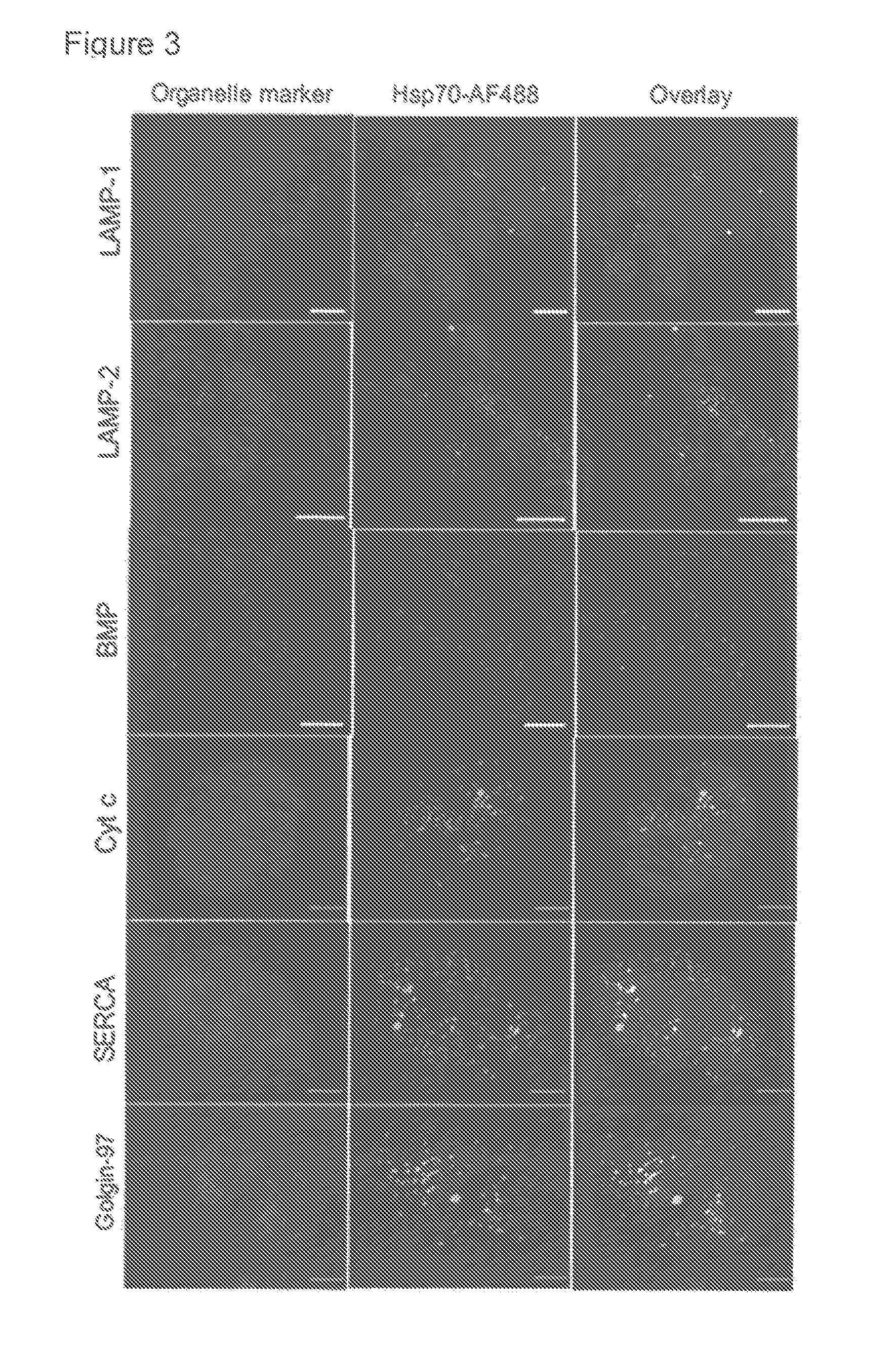
Methods for increasing intracellular activity of Hsp70
ActiveUS9662375B2Reduce the amount of solutionFacilitate the uptake of enzymesBiocidePeptide/protein ingredientsGlycoprotein metabolismLysosomal enzyme defect
Owner:KEMPHARM DENMARK AS
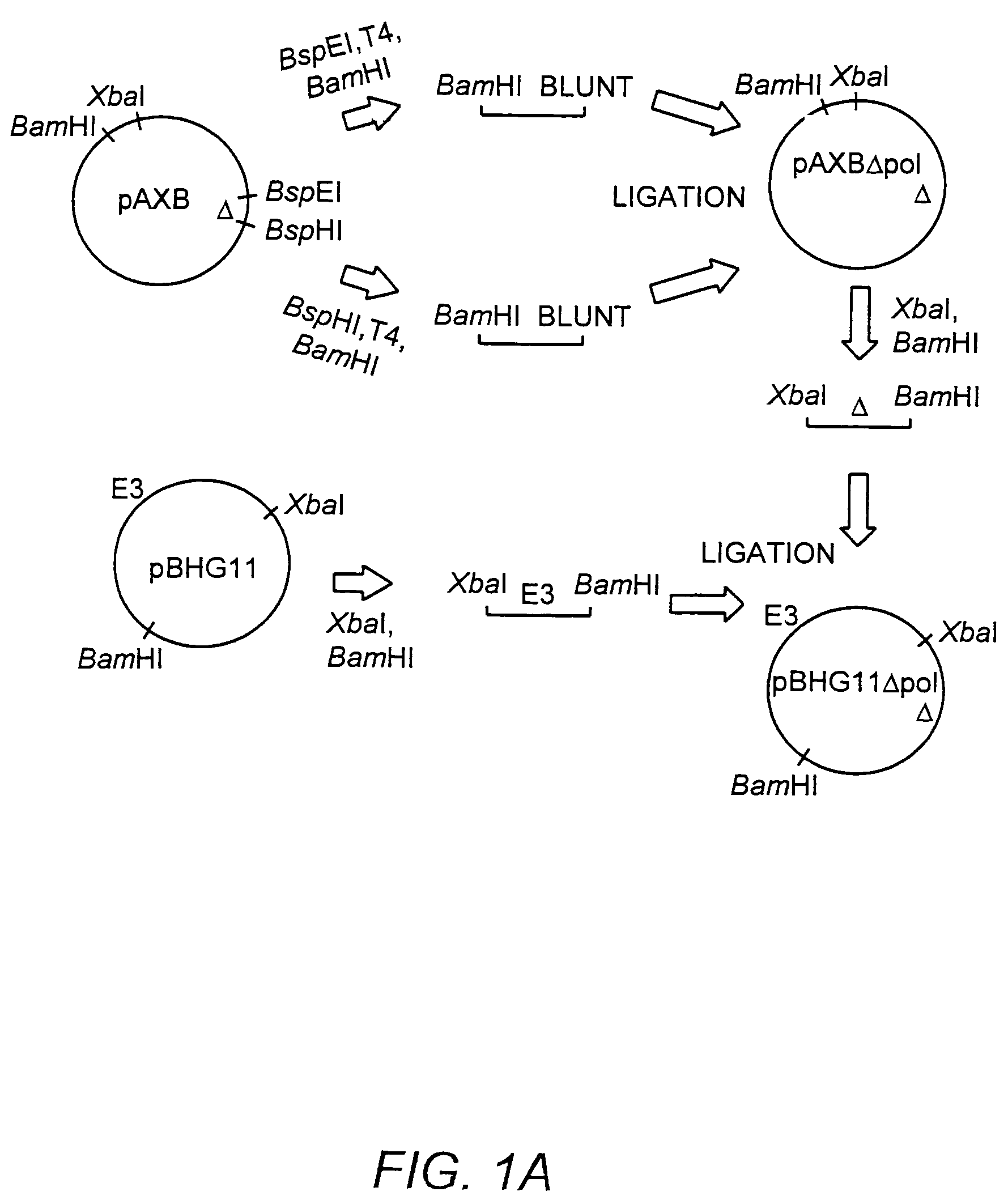
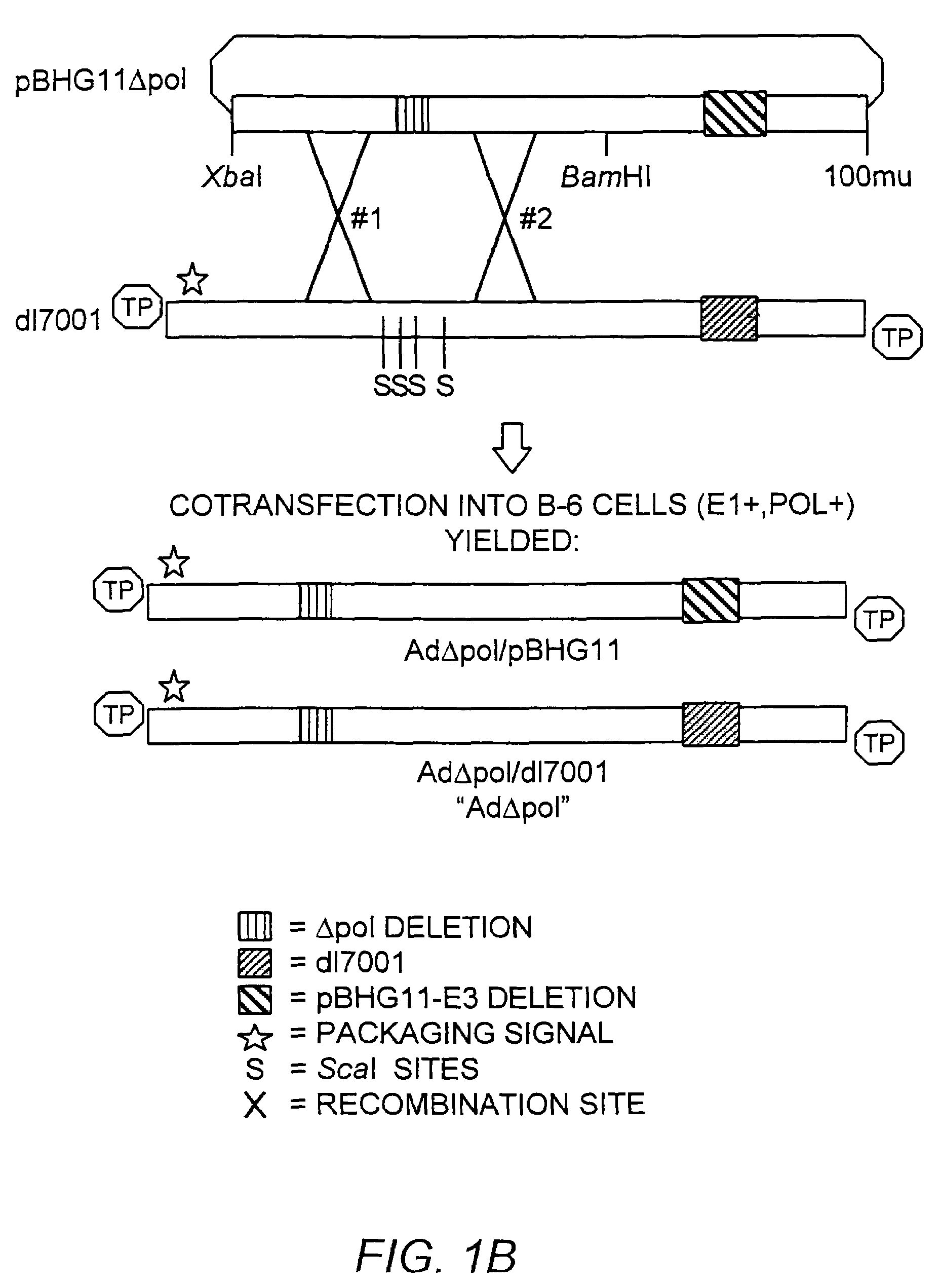
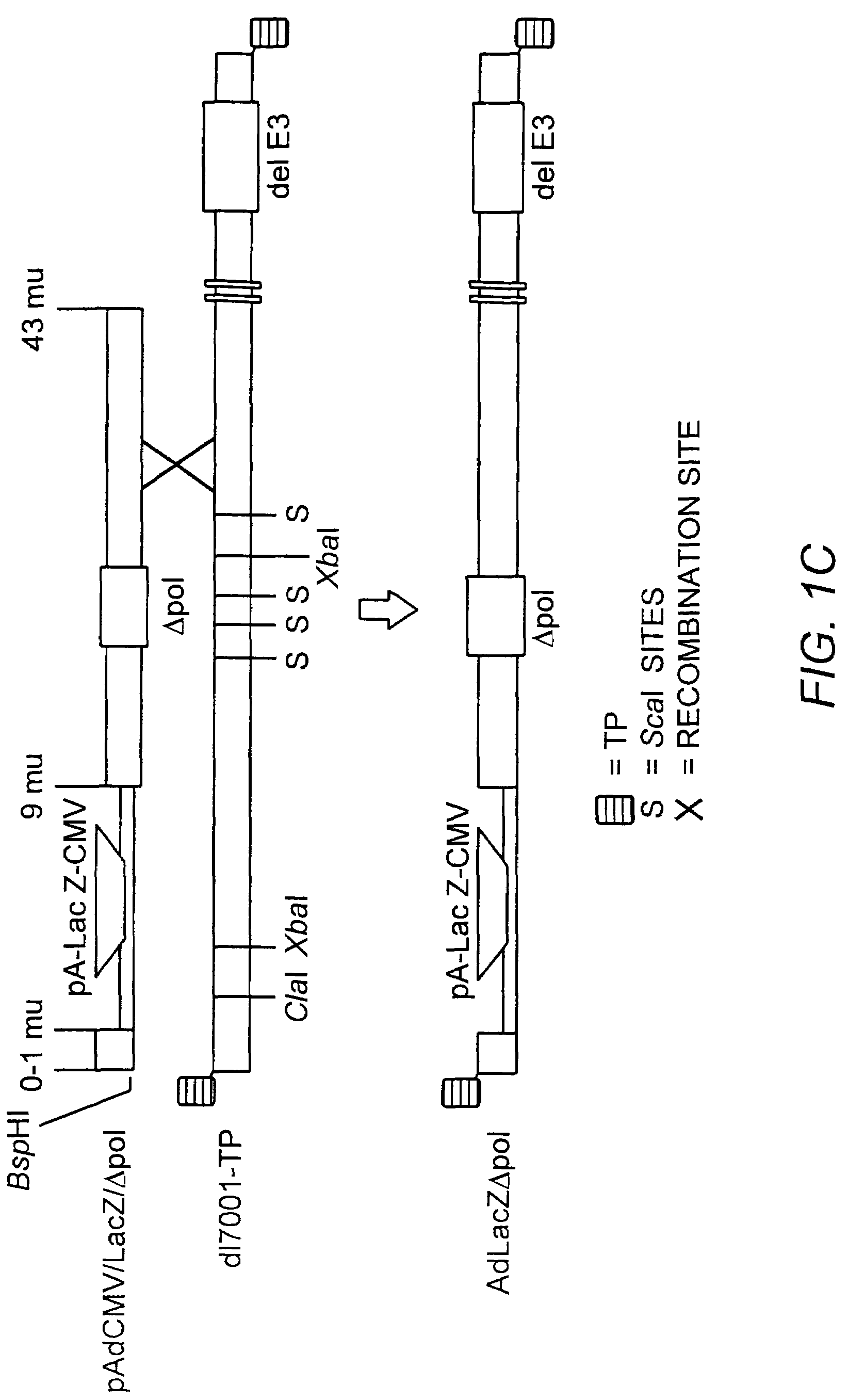
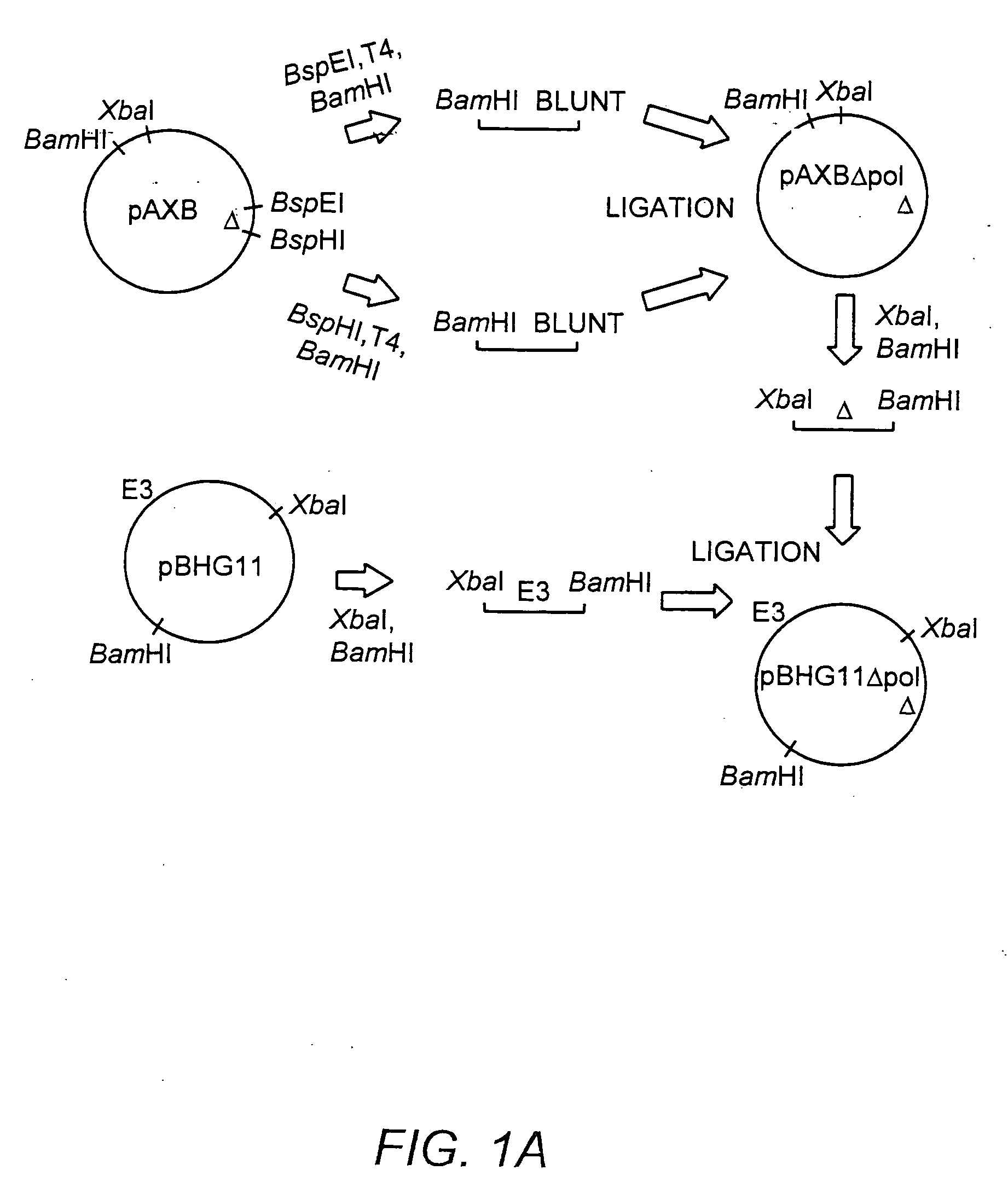
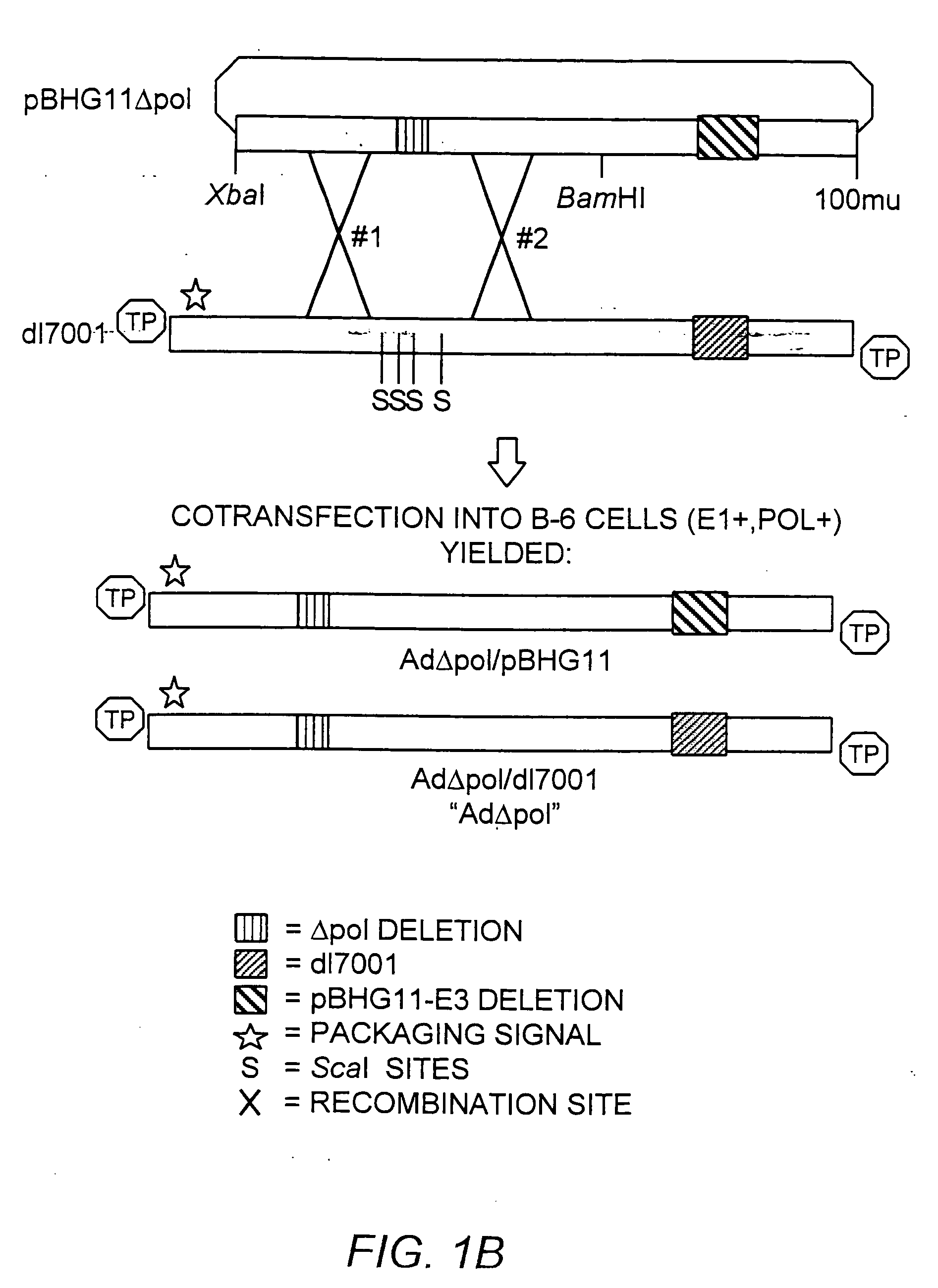
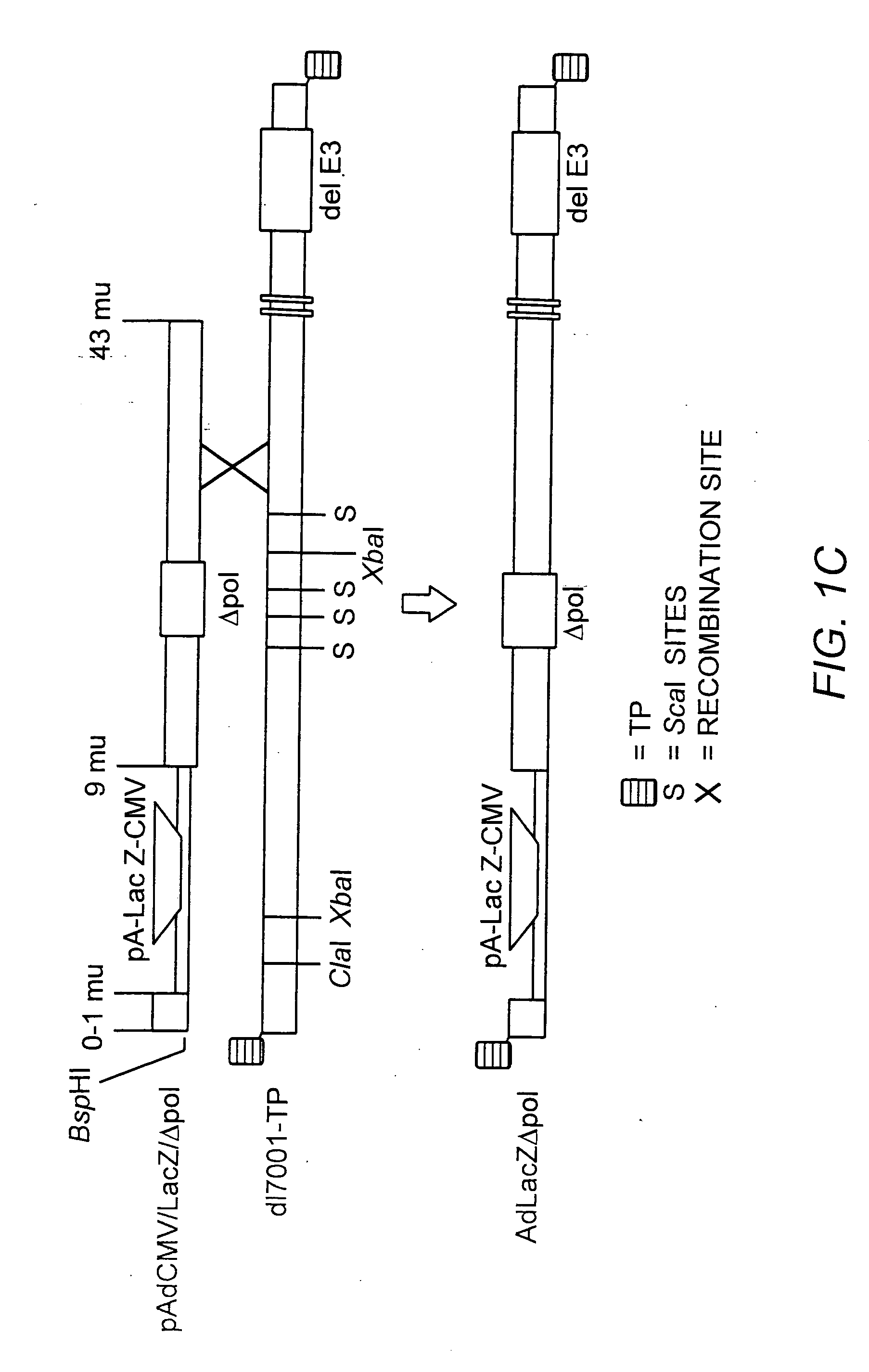
Deleted adenovirus vectors and methods of making and administering the same
The present invention provides deleted adenovirus vectors. The inventive adenovirus vectors carry one or more deletions in the IVa2, 100K, polymerase and / or preterminal protein sequences of the adenovirus genome. The adenoviruses may additionally contain other deletions, mutations or other modifications as well. In particular preferred embodiments, the adenovirus genome is multiply deleted, i.e., carries two or more deletions therein. The deleted adenoviruses of the invention are “propagation-defective” in that the virus cannot replicate and produce new virions in the absence of complementing function(s). Preferred adenovirus vectors of the invention carry a heterologous nucleotide sequence encoding a protein or peptide associated with a metabolic disorder, more preferably a protein or peptide associated with a lysosomal or glycogen storage disease, most preferably, a lysosomal acid α-glucosidase. Further provided are methods for producing the inventive deleted adenovirus vectors. Further provided are methods of administering the deleted adenovirus vectors to a cell in vitro or in vivo.
Owner:DUKE UNIV
Methods for increasing intracellular activity of hsp70
ActiveUS20130230506A1Safeguarding lysosomal integrityReduce the amount of solutionBiocidePeptide/protein ingredientsGlycoprotein metabolismLysosomal enzyme defect
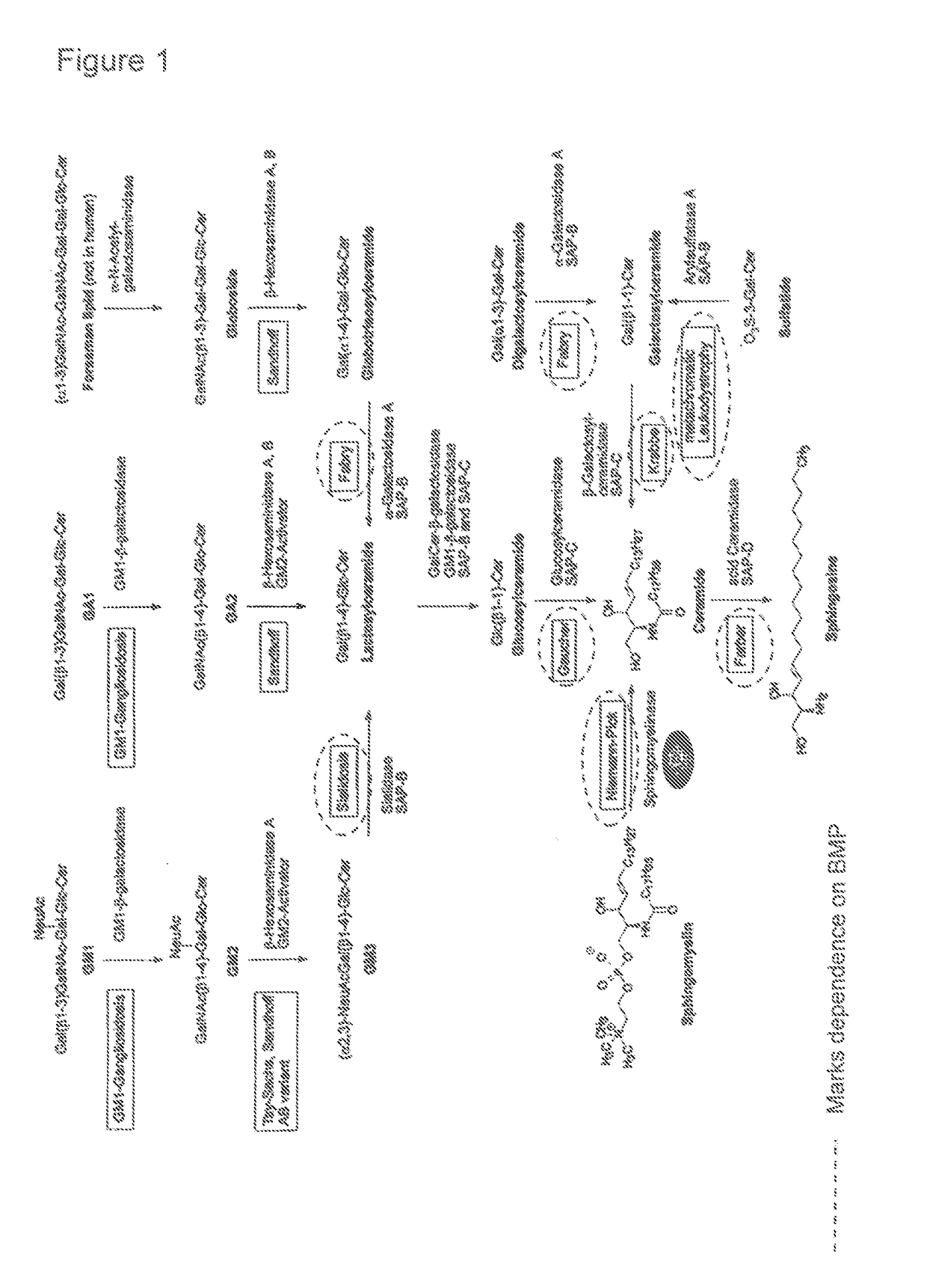
The present invention relates to a bioactive agent capable of increasing the intracellular concentration and / or activity of Hsp70 for use in the treatment of a lysosomal storage disease which arise from a defect in an enzyme whose activity is not directly associated with the presence of lysosomal BMP as a co-factor; such as glycogen storage diseases, gangliosidoses, neuronal ceroid lipofuscinoses, cerebrotendinous cholesterosis, Wolman's disease, cholesteryl ester storage disease, disorders of glycosaminoglycan metabolism, mucopolysaccharidoses, disorders of glycoprotein metabolism, mucolipidoses, aspartylglucosaminuria, fucosidosis, mannosidoses, and sialidosis type II.
Owner:KEMPHARM DENMARK AS
Primer combination, method and kit for constructing targeted library of multiple inherited metabolic liver diseases based on high-throughput sequencing
ActiveCN109468372ASave time and costEasy to operateMicrobiological testing/measurementLibrary creationExonLiver disease
The invention discloses a primer combination, method and kit for constructing a targeted library of multiple inherited metabolic liver diseases based on high-throughput sequencing. The base sequence of the primer combination is shown as SEQ ID No. 1 to SEQ ID No. 2245, and the primer combination covers total 703 exons and cutting regions of 43 genes such as SERPINA1, G6PC, SLC37A4, AGL and the like, and is capable of detecting 41 sub-type gene variation loci of totally 20 inherited metabolic liver diseases comprising alpha-1-antitrypsin deficiency, glycogen storage disease, citrullinemia, aminosuccinuria, hemochromatosis, porphyrinopathy, cystic fibrosis, bile acid synthesis defect, Gaucher disease, hereditary fructose intolerance, cholesterol ester storage disease, Gilbert syndrome, Dubinsyndrome, Rotor syndrome, progressive familial intrahepatic choleatasia and the like. The primer combination disclosed by the invention is simple in operation, high in accuracy and low in time consumption, and the time cost and manual cost used for detecting genes and fragments one by one during Sanger sequencing can be greatly saved.
Owner:THE FIRST AFFILIATED HOSPITAL OF ARMY MEDICAL UNIV
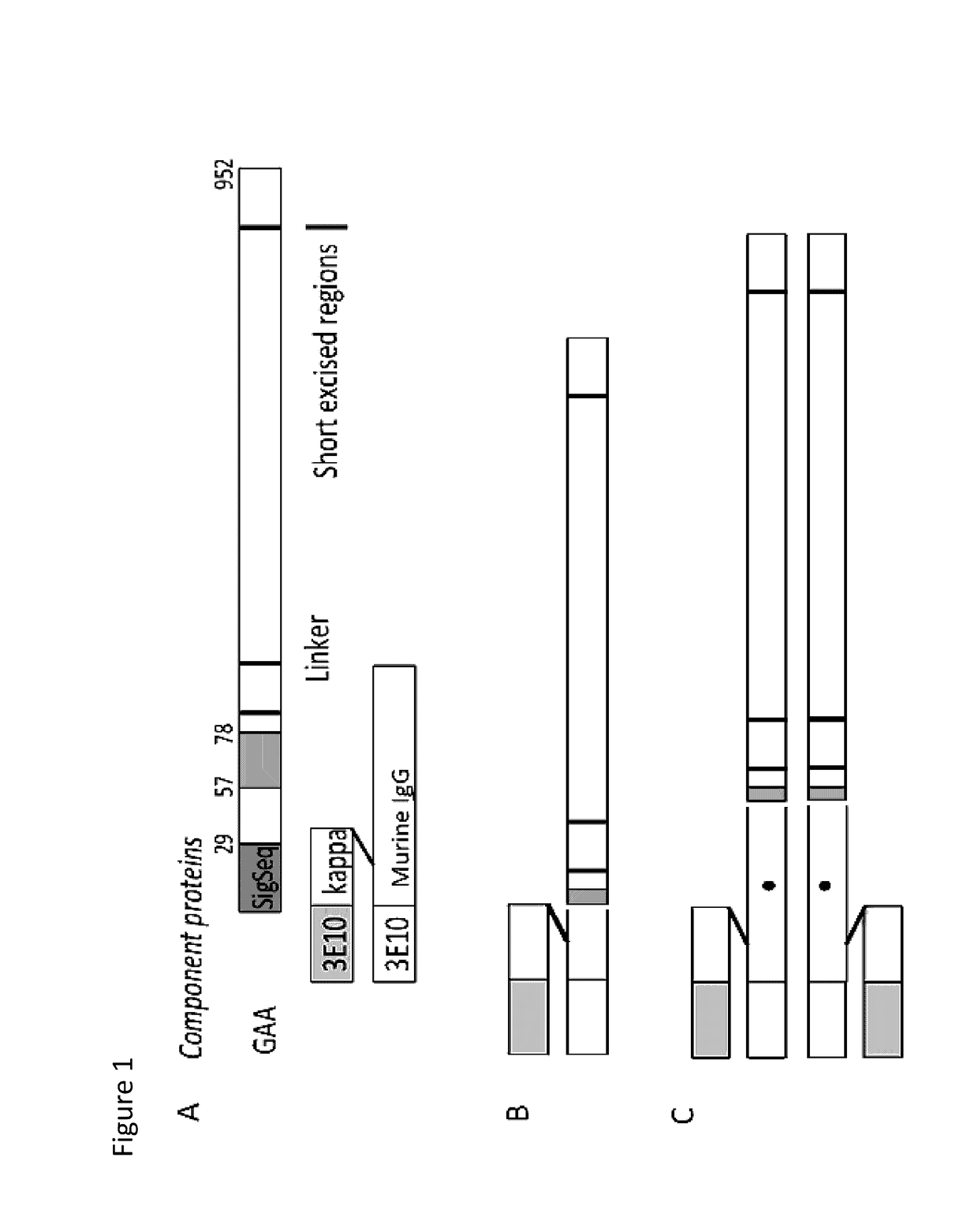
Methods and compositions for treatment of glycogen storage diseases and glycogen metabolism disorders
ActiveUS20170130216A1Reduce deliveryDecreasing glycogen accumulationPolypeptide with localisation/targeting motifPeptide/protein ingredientsAcid alpha-glucosidaseGlycogen storage disease type Ia
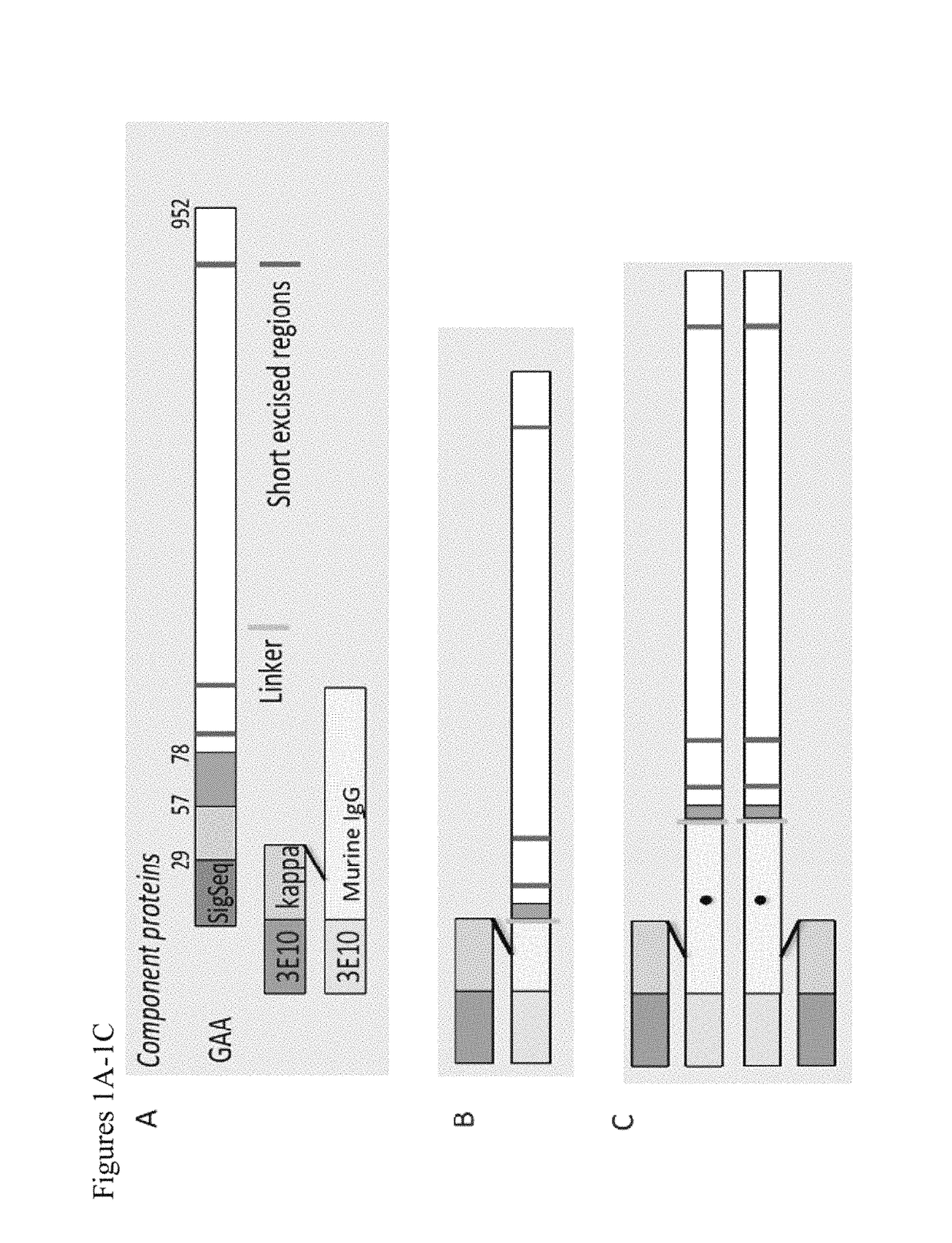
The present disclosure provides for compositions comprising a chimeric polypeptide comprising a polypeptide effective for treating glycogen storage disease and an internalizing moiety that promotes delivery into cells. In certain embodiments, the polypeptide effective for treating glycogen storage disease is an acid alpha-glucosidase (GAA), a laforin, an amyloglucosidase (AGL), a malin, or an alpha amylase. The present disclosure also provides for methods for decreasing glycogen accumulation in cells or for treating glycogen storage diseases, including Forbes-Cori Disease, Andersen Disease, von Gierke Disease, Pompe Disease, and Lafora Disease, comprising administering the chimeric polypeptide disclosed herein.
Owner:VALERION THERAPEUTICS
Piperazine derivatives as trpml modulators
ActiveUS20190248764A1Reduce severityNervous disorderOrganic chemistryDuchenne muscular dystrophyMuscular dystrophy
The new piperazine derivatives are modulators of TRPML and are useful in treating disorders related to TRPML activities such as lysosome storage diseases, muscular dystrophy, age-related common neurodegenerative diseases, oxidative stress or reactive oxygen species (ROS) related diseases, and ageing.
Owner:LYSOWAY THERAPEUTICS INC
New application of recombinant human thymosin alpha collagens
The invention discloses a new application of recombinant human thymosin alpha collagens, which relates to a recombinant human thymosin alpha collagen. The invention also provides the application of the recombinant human thymosin alpha collagen in preparing anti-fatigue drugs. The recombinant human thymosin alpha collagen (referred to as thymosin alpha collagen) refers to a recombinant human thymosin alpha collagen and a tissue separated and purified thymosin alpha collagen. The effect of the thymosin alpha collagen in various anti-fatigues is implemented through increasing the glycogen storage of the liver and muscles and reducing the level of serum urea nitrogen so as to improve the fatigue resistance.
Owner:XIAMEN UNIV
Prevention And Treatment Of Diseases Caused By Elevated Levels Of Deoxy-Sphingolipids
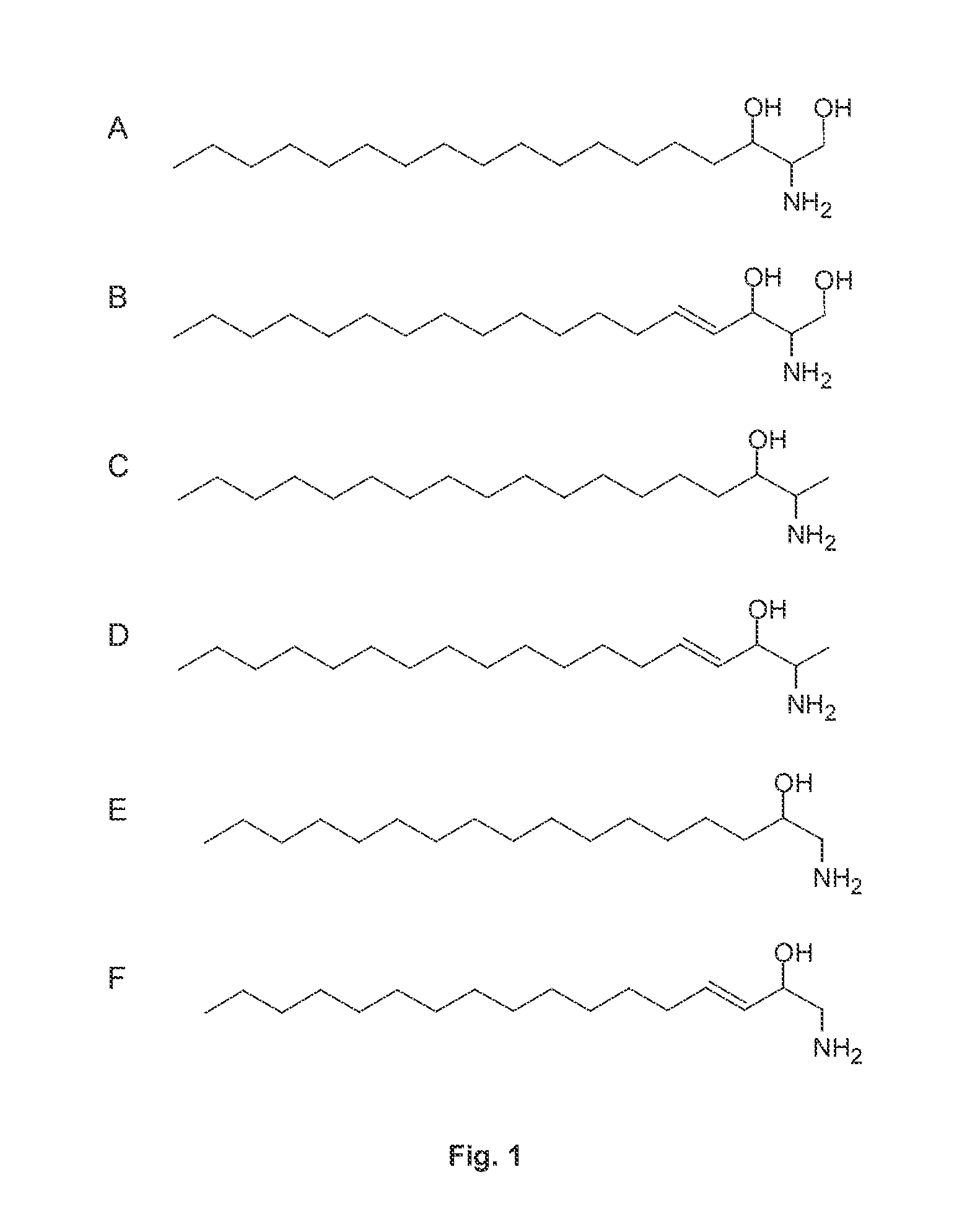
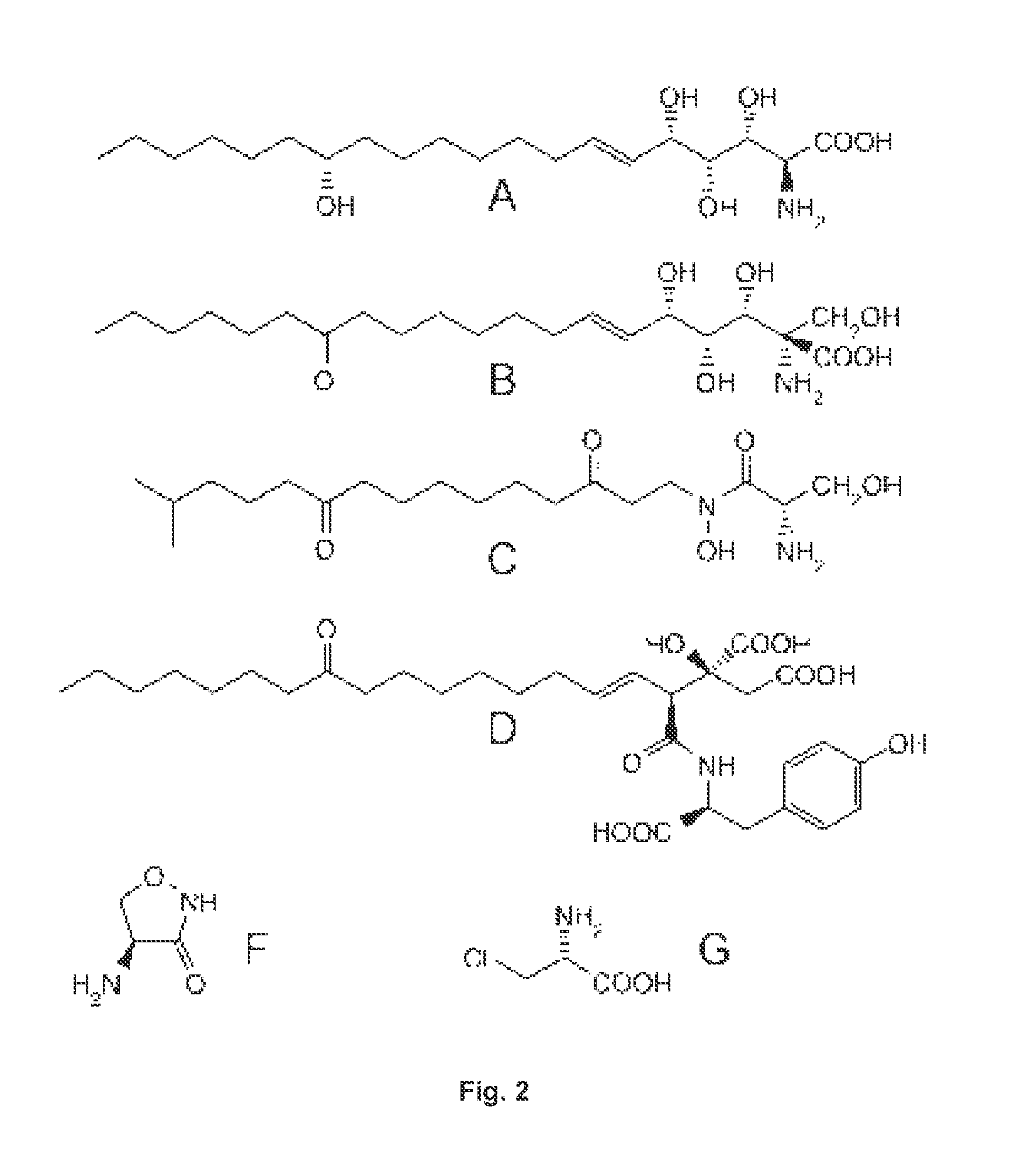
Substances and methods of use of substances capable of inhibiting serine-palmitoyltransferase (SPT) and / or capable of competing with L-alanine and glycine, including in the reaction catalysed by SPT, including L-serine and D-serine and other compounds, to suppress cytotoxic sphingolipid metabolites, in particular deoxy-sphingolipids. The substances and methods can be used to prevent and treat disease caused by or associated with elevated levels of deoxy-sphingolipids, namely, diabetes (type 1 and type 2 diabetes), particularly diabetic neuropathy, neurodegenerative diseases such as hereditary and sensory neuropathy type I (HSAN1), amyotrophic lateral sclerosis (ALS), Alzheimer disease, other neurological disorders (e.g. depressive disorders, schizophrenia), medication-induced neuriopathies (e.g. induced by treatment with cytostatics like paclitaxel, cis-platin compounds etc.) and other metabolic disorders such as glycogen storage disease type 1a and asthma.
Owner:UNIV ZURICH
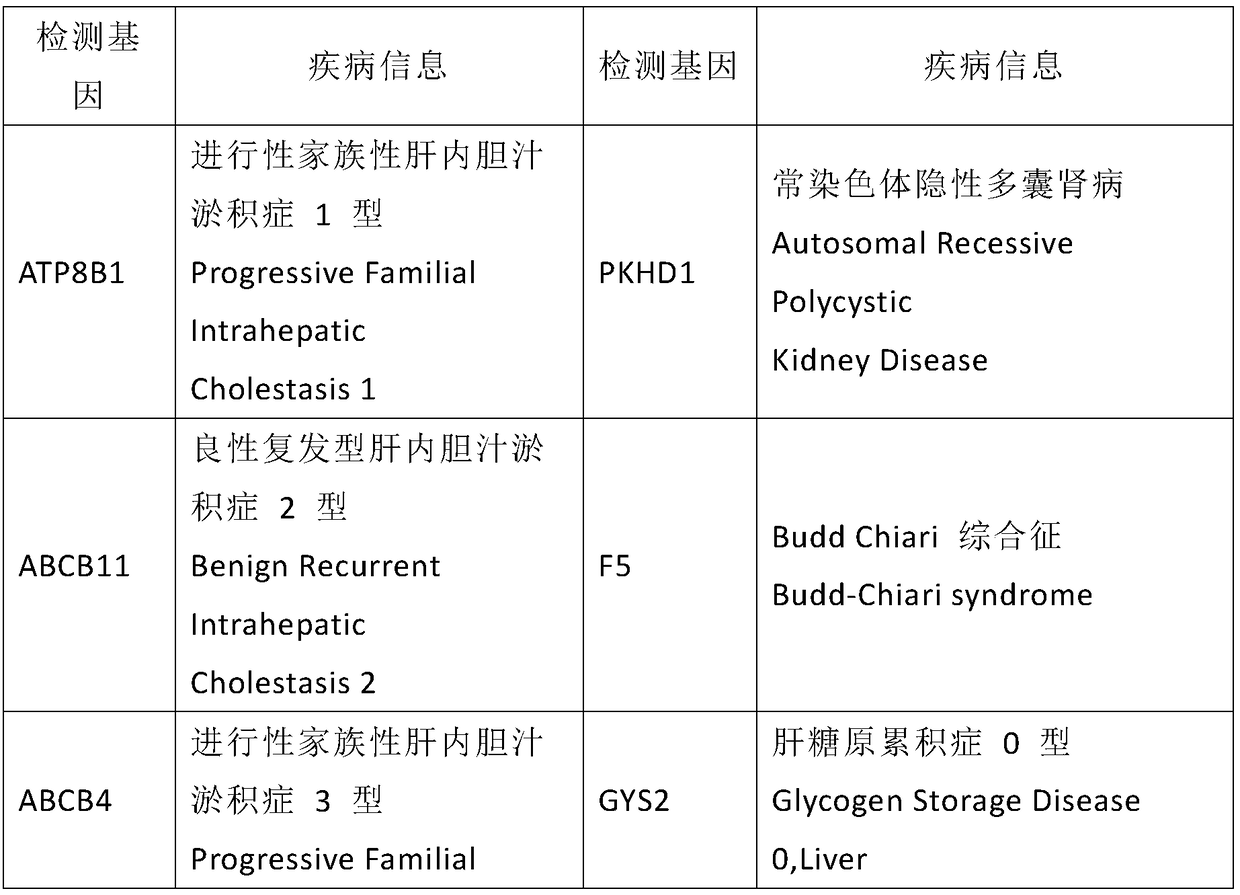
Kit used for screening genetic liver diseases
ActiveCN108913761AInterpretation is clear and objectiveHigh detection throughputMicrobiological testing/measurementNPC1Autosomal Recessive Polycystic Kidney Disease
The invention discloses a kit used for screening genetic liver diseases. The kit is high in detection flux, sensitivity, and specificity. The genetic liver diseases comprise progressive familial intrahepatic cholestasis, benign recurrent intrahepatic cholestasis, congenital bile acid synthesis defect, idiopathic bile acid malabsorption, Dubin-Johnson syndrome, hereditary hemochromatosis, alpha1 antitrypsin deficiency, glycogen storage disease, autosomal recessive polycystic kidney disease, Budd-Chiari syndrome, and the like. 39 genes, including ATP8B1, ABCB11, ABCB4, TJP2, NR1H4, HSD3B7, AKR1D1, CYP7B1, AMACR, ABCD3, SLC10A2, ABCC2, HFE, TFR2, SERPINA1, G6PC, SLC37A4, AGL, PYGL, SLC40A1, PKHD1, F5, GYS2, VIPAS39, SLC25A13, JAG1, NOTCH2, PHKA2, PHKB, PHKG2, PHKA1, ALAD, HAMP, HFE2, SMPD1, ATP7B, ABCA1, NPC2, and NPC1, are detected in screening of genetic liver diseases. The kit comprises targeting capture probes SEQ NO:1-SEQ NO:722 targeting at all the exons of the 39 genes, and the kitis used for gene detection.
Owner:施军平 +1
Method of treating glycogen storage disease
Owner:VIKING THERAPEUTICS INC
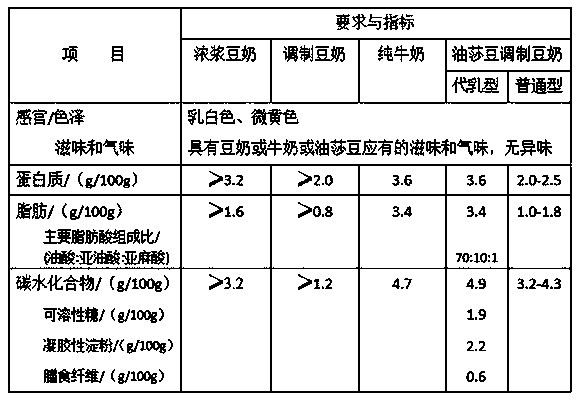
Cyperus esculentus blended soymilk and producing method thereof
The invention discloses cyperus esculentus blended soymilk and a producing method thereof. The cyperus esculentus blended soymilk is prepared from soybeans, fresh cyperus esculentus, white granulatedsugar, monoglycerol and diglycerol fatty acid ester and water, and a production technological process of the cyperus esculentus blended soymilk comprises steps of raw material pretreatment, wet superfine grinding, blending, volume metering, homogenizing, filtering, ultrahigh-temperature instantaneous sterilization and sterile filling. The cyperus esculentus blended soymilk is an optimal combination of cyperus esculentus or cyperus esculentus pulp, soybeans or a soybean protein powder and single plant milk in aspects of components, effects, characters and taste. The nutrition is richer, more comprehensive and more balanced; there is no or less sugar and thickening agent; and the soymilk is rich in nutrition, natural, fragrant, sweet and pleasant in taste and more satisfactory, is beneficialto prevention and treatment of metabolic syndromes such as diabetes, a glycogen storage disease, obesity and the like, has no or few side effects such as abdominal distension and diarrhea caused by drinking of the soymilk by people with dyspepsia, hiccup and gastrointestinal dysfunction, and is novel milk replacer plant milk which is suitable for higher nutritional and healthy requirements of wider crowds.
Owner:陈世金
Compositions and uses thereof
ActiveUS8507462B2Improved release profileBiocideOrganic active ingredientsHypoglycaemic seizureSerum ige
Methods of controlling serum glucose levels in an individual are described, the methods including the step of administering to said individual a therapeutic food composition comprising a waxy and / or hydrothermally treated starch. The method may be used to treat or prevent hypoglycaemia in patients susceptible to hypoglycaemic episodes, for example patients with glycogen storage disease, diabetes or liver disease. The method may also be used in sports nutrition. Also described are compositions for use in the methods.
Owner:GLYCOLOGIC LIMITED GLASGOW CALEDONIAN UNIVERSITY
Traditional Chinese medicine composition with function of adjuvant improvement in kidney function of chronic kidney disease patient
ActiveCN107441204AIt has the function of invigorating kidney and QiImprove kidney functionUrinary disorderLeech/worm material medical ingredientsChannel tunnelTubulointerstitial fibrosis
The invention belongs to the field of application of traditional Chinese medicines to adjuvant improvement in the treatment of a kidney function of a chronic kidney disease patient. Renal tubulointerstitial fibrosis is a common pathological process from CKD progress caused by various disease causes to end-stage renal failure, and the anti-renal fibrosis treatment becomes an important link of preventing and delaying the CKD progress. A traditional Chinese medicine composition with a function of adjuvant improvement in the kidney function of the chronic kidney disease patient consists of the following components in parts by weight: 200-600 parts of astragalus membranaceus, 200-600 parts of radix polygonum multiflorum preparata, 100-300 parts of prepared rhubarb, 100-300 parts of ligusticum wallichii, 10-120 parts of leech particles, 200-600 parts of charred fructus crataegi, 200-800 parts of exocarpium benincasae, 100-400 parts of peach kernels, 100-600 parts of centella asiatica and 100-300 parts of herba lycopi. In the formula, the astragalus membranaceus and the radix polygonum multiflorum preparata as monarchs have the efficacies of benefiting Qi and tonifying kidney; the leech particles as ministers have the efficacies of breaking glycogen storage disease, removing blood stasis, promoting blood circulation and prmoting diuresis through channel tunnels; the peach kernels, the ligusticum wallichii, theherba lycopi and the fructus crataegi have the efficacy of promoting blood circulation to remove blood stasis; the prepared rhubarb has the efficacy of dispelling caramelized, damp and turbid blood stasis; the exocarpium benincasae, the herba lycopi and the centella asiatica as adjuvant medicines have the efficacies of dissipating diuresis and dampness. Experiments and clinical trials show that the traditional Chinese medicine composition has the effect of adjuvant treatment on renal insufficiency.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
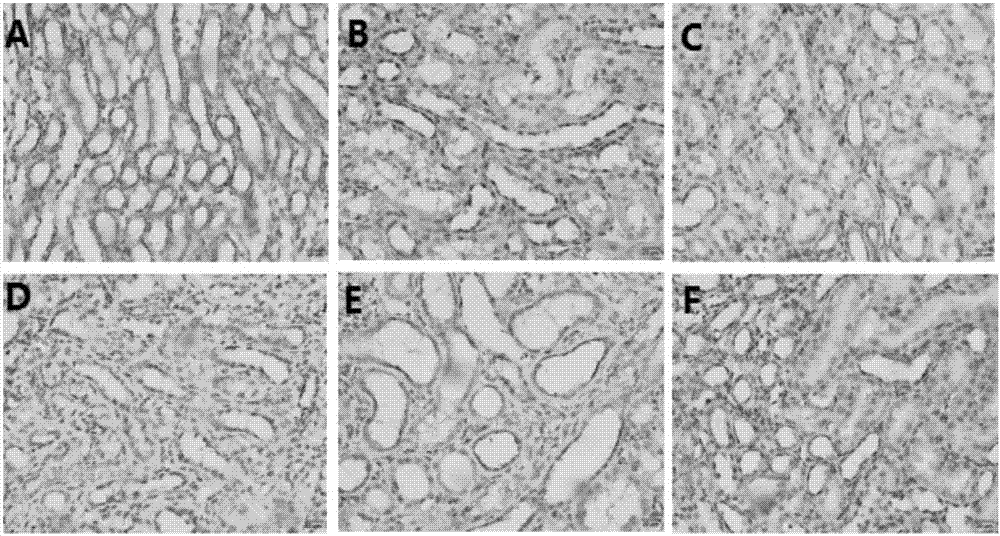
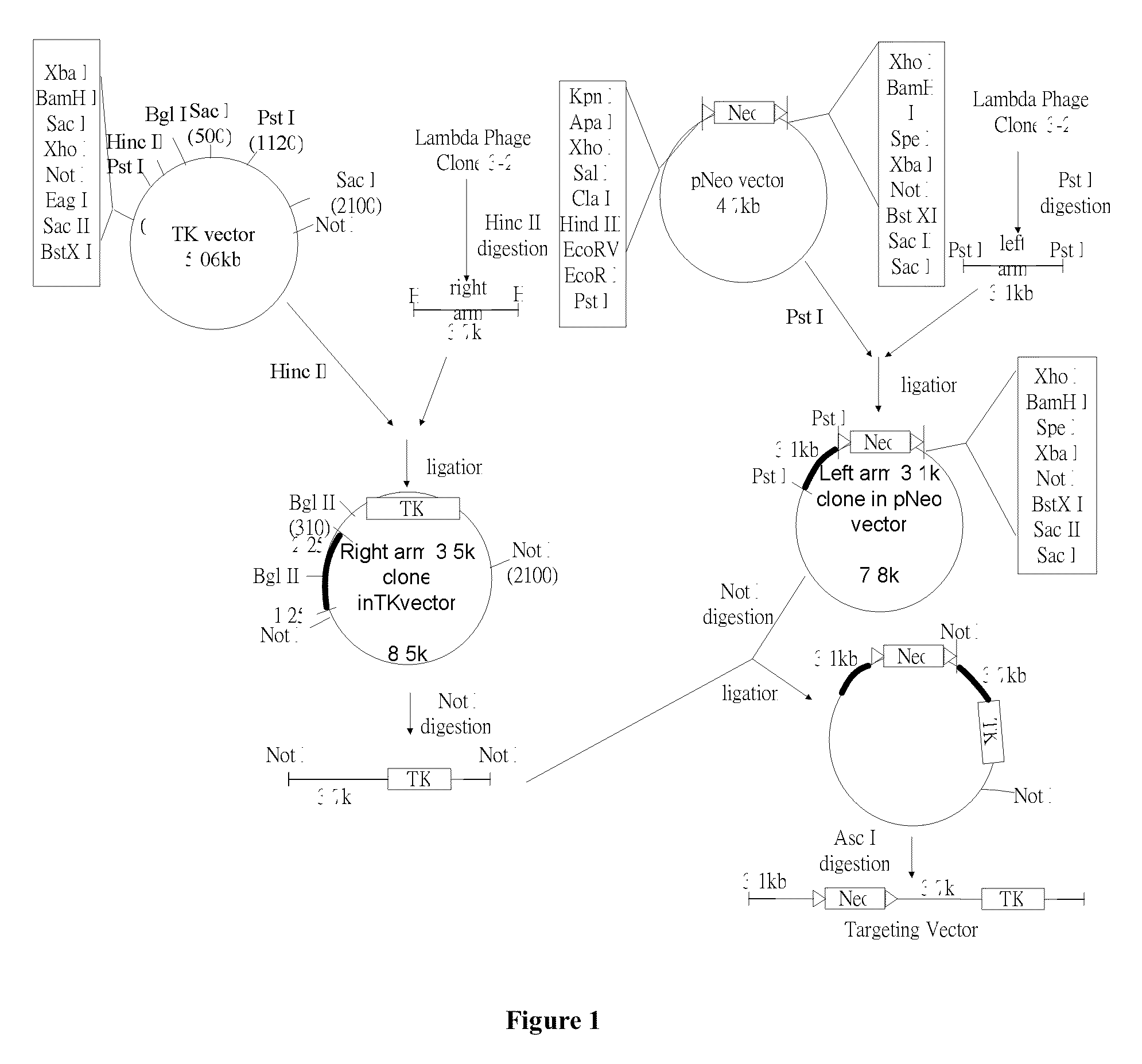
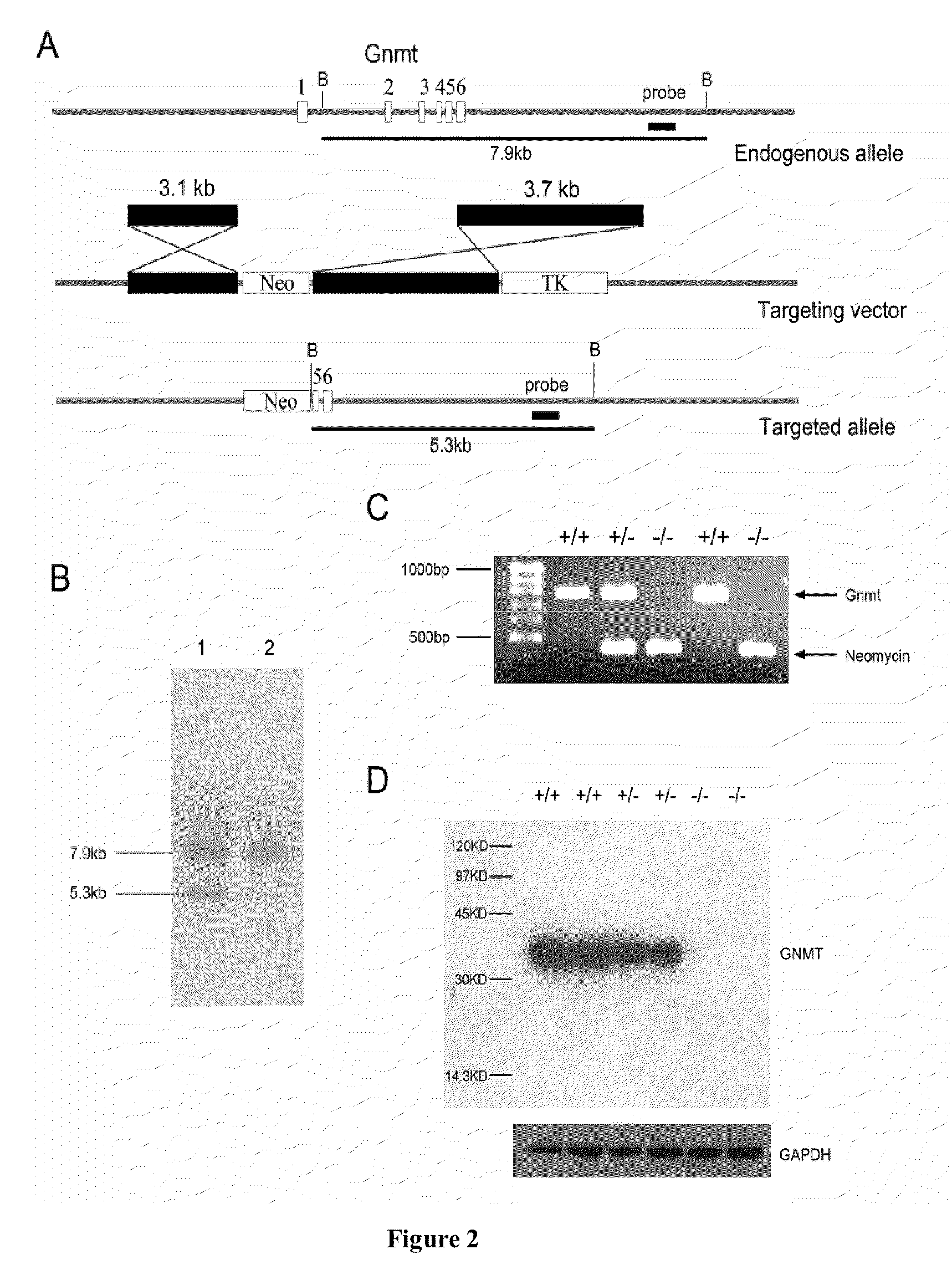
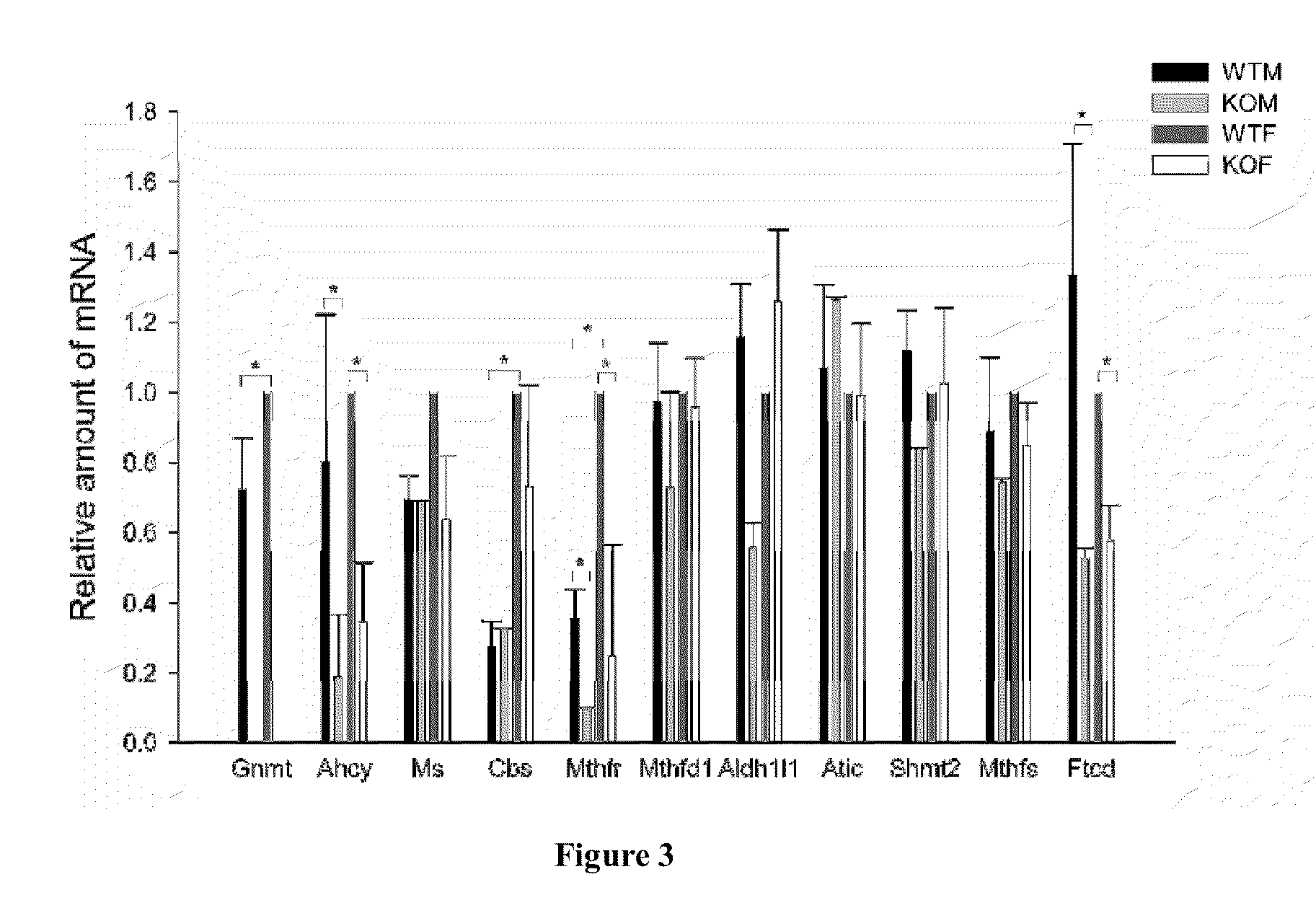
Glycine N-methyltransferase (GNMT) Animal model and use thereof
InactiveUS20090148428A1Guaranteed accuracyOrganic active ingredientsPeptide/protein ingredientsFatty liverHepatocellular carcinoma
The present invention is a new type of Glycine N-methyltransferase (GNMT) knockout mice model. This model can be applied to screen drug, test of treatment and search for diagnostic marker of hepatocellular carcinoma (HCC), glycogen storage disease, liver dysplasia, fatty liver and other liver disease.
Owner:NATIONAL YANG MING UNIVERSITY
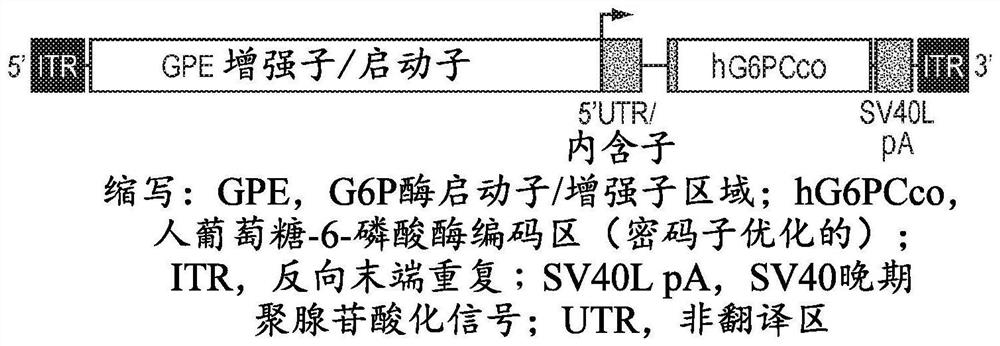
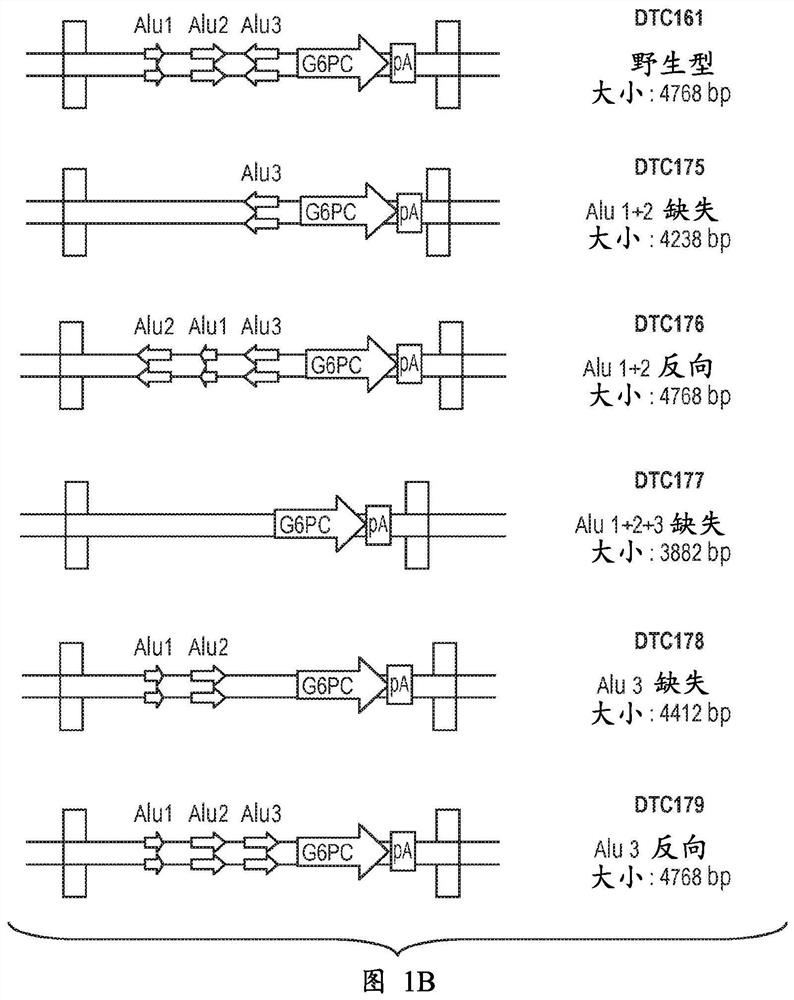
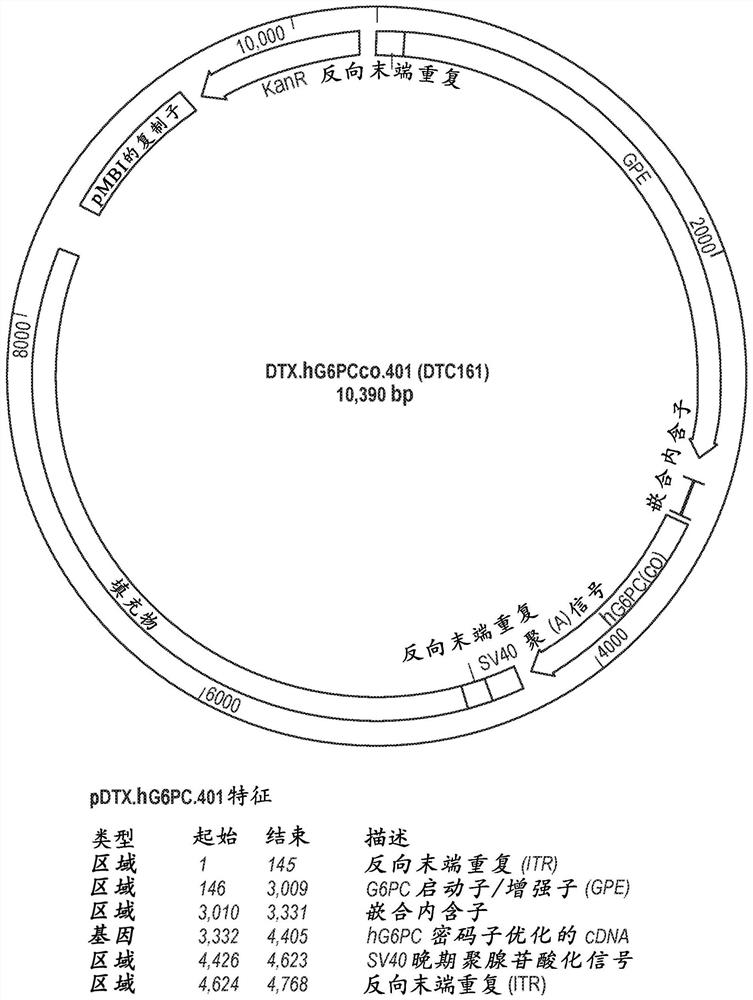
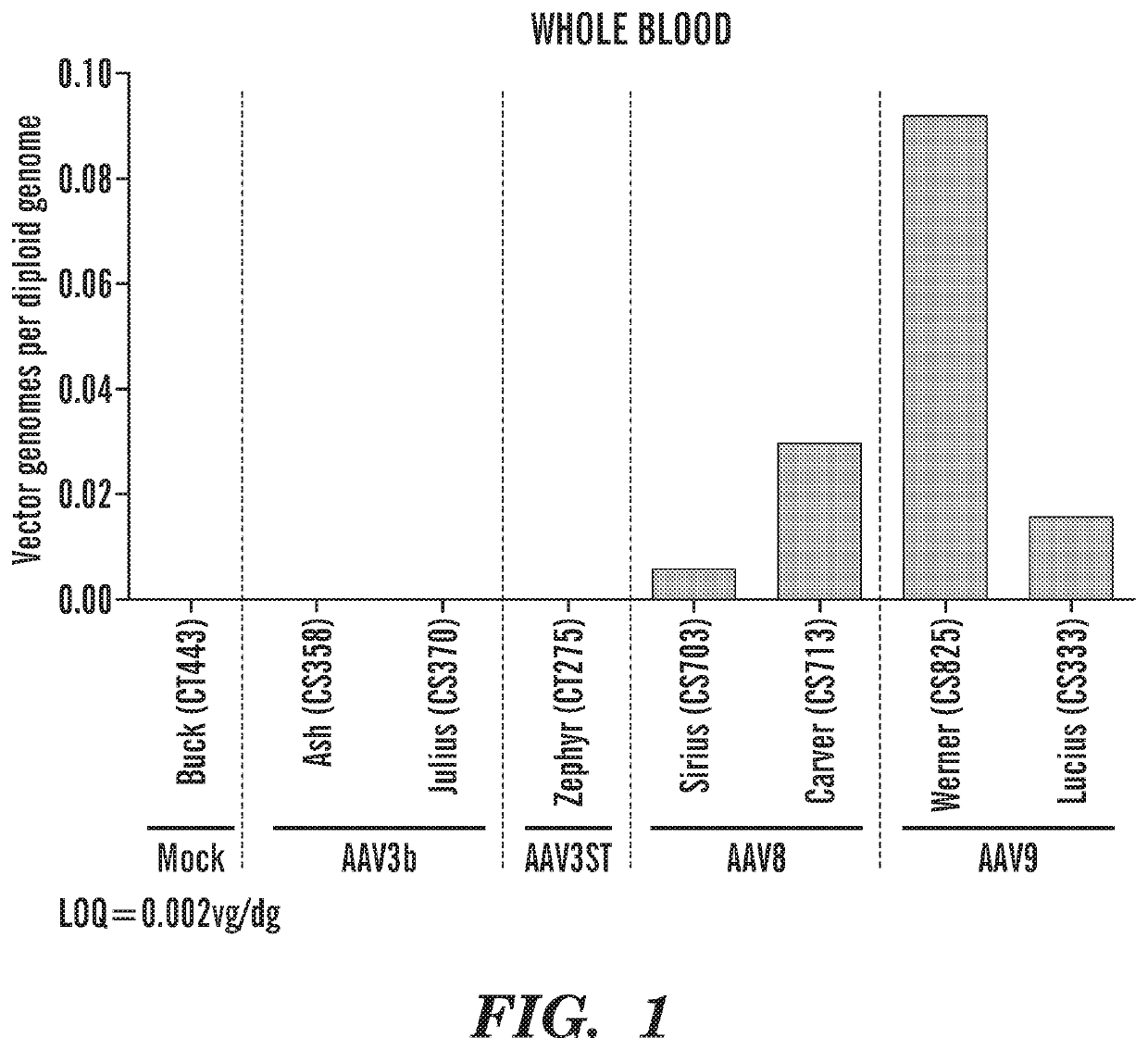
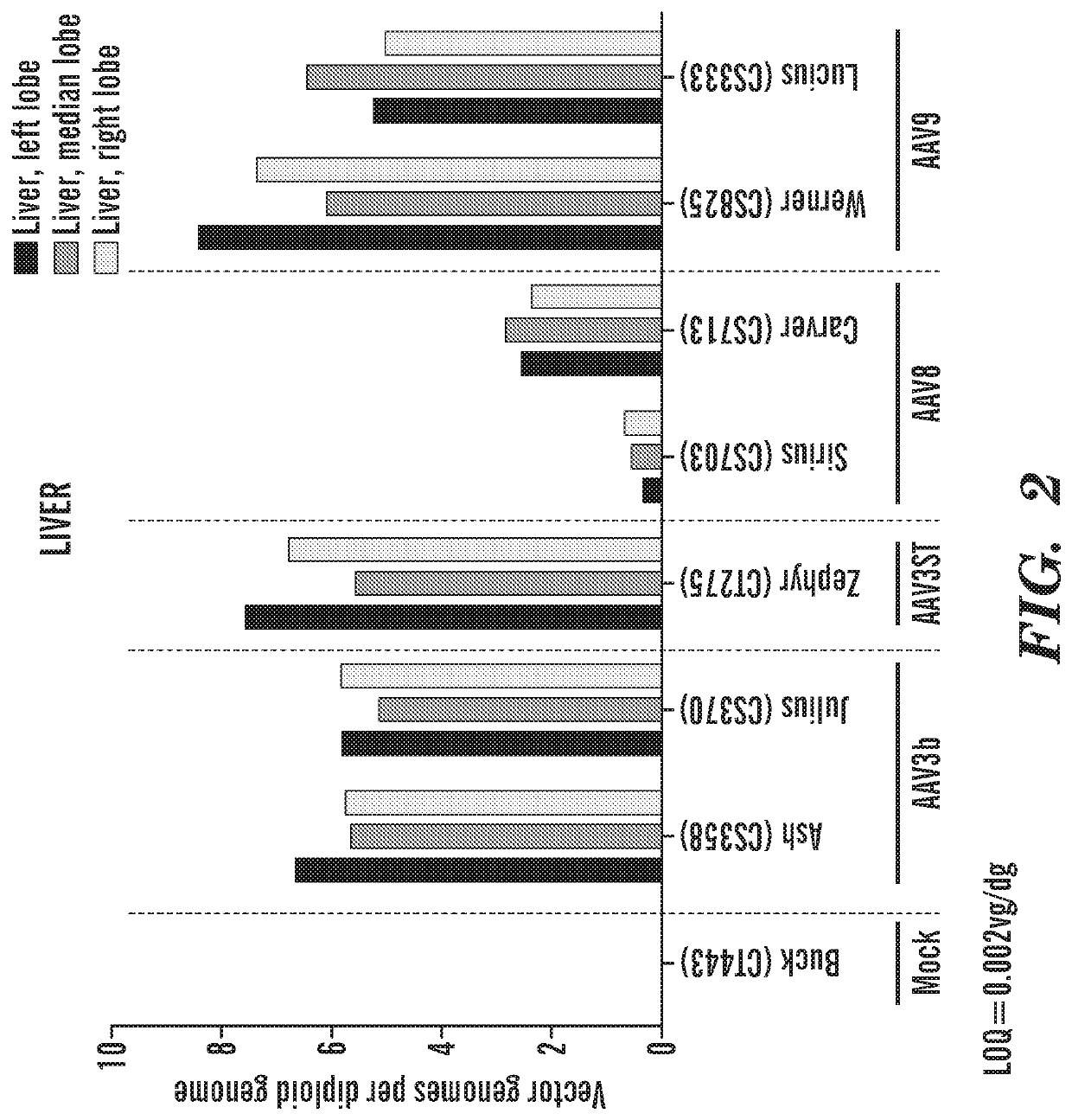
Adeno-associated virus vectors encoding modified g6pc and uses thereof
ActiveCN107636153AImprove balanceSuppress hypoglycemiaPeptide/protein ingredientsMetabolism disorderGlucosephosphataseHydrolase
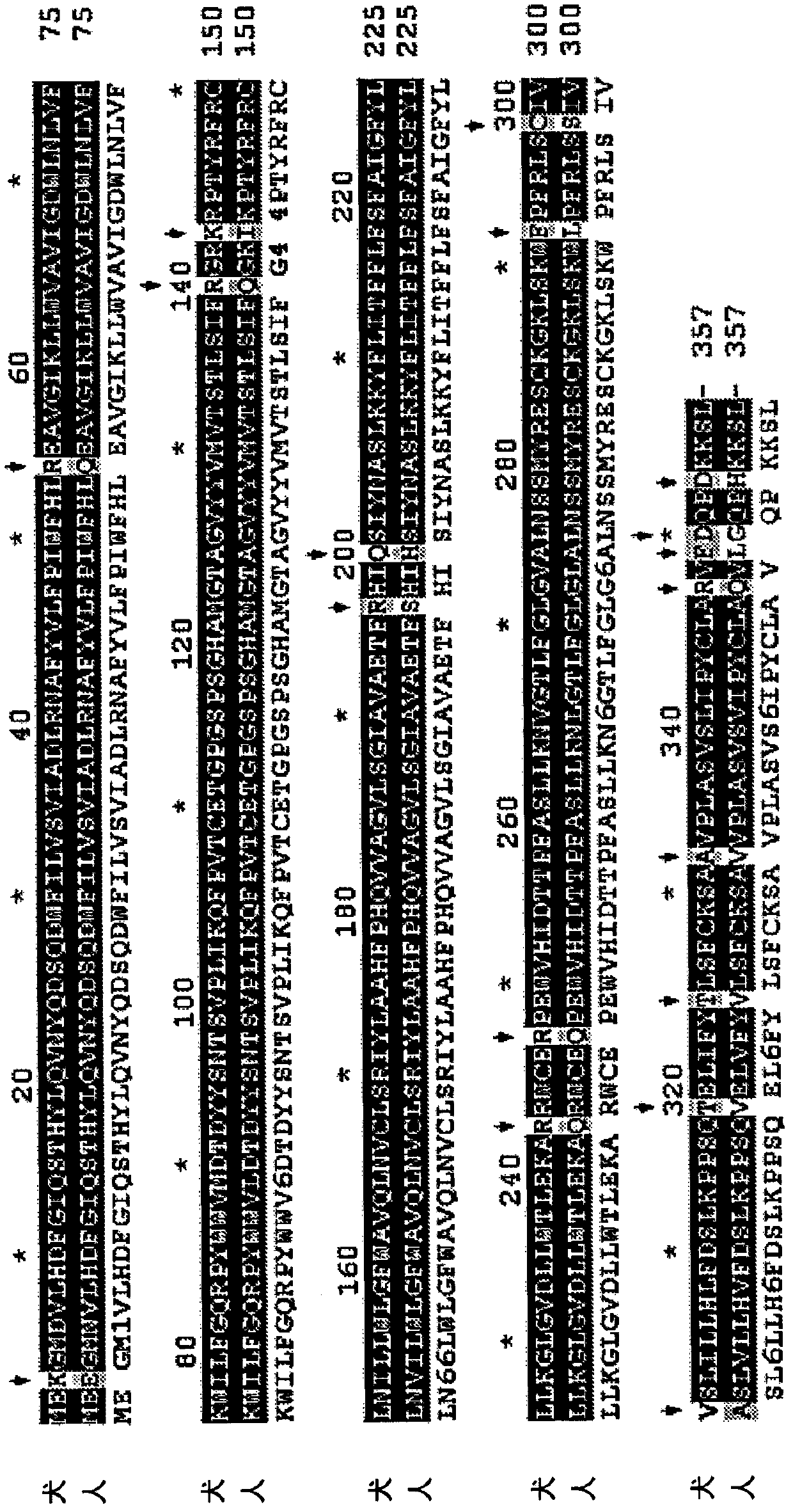
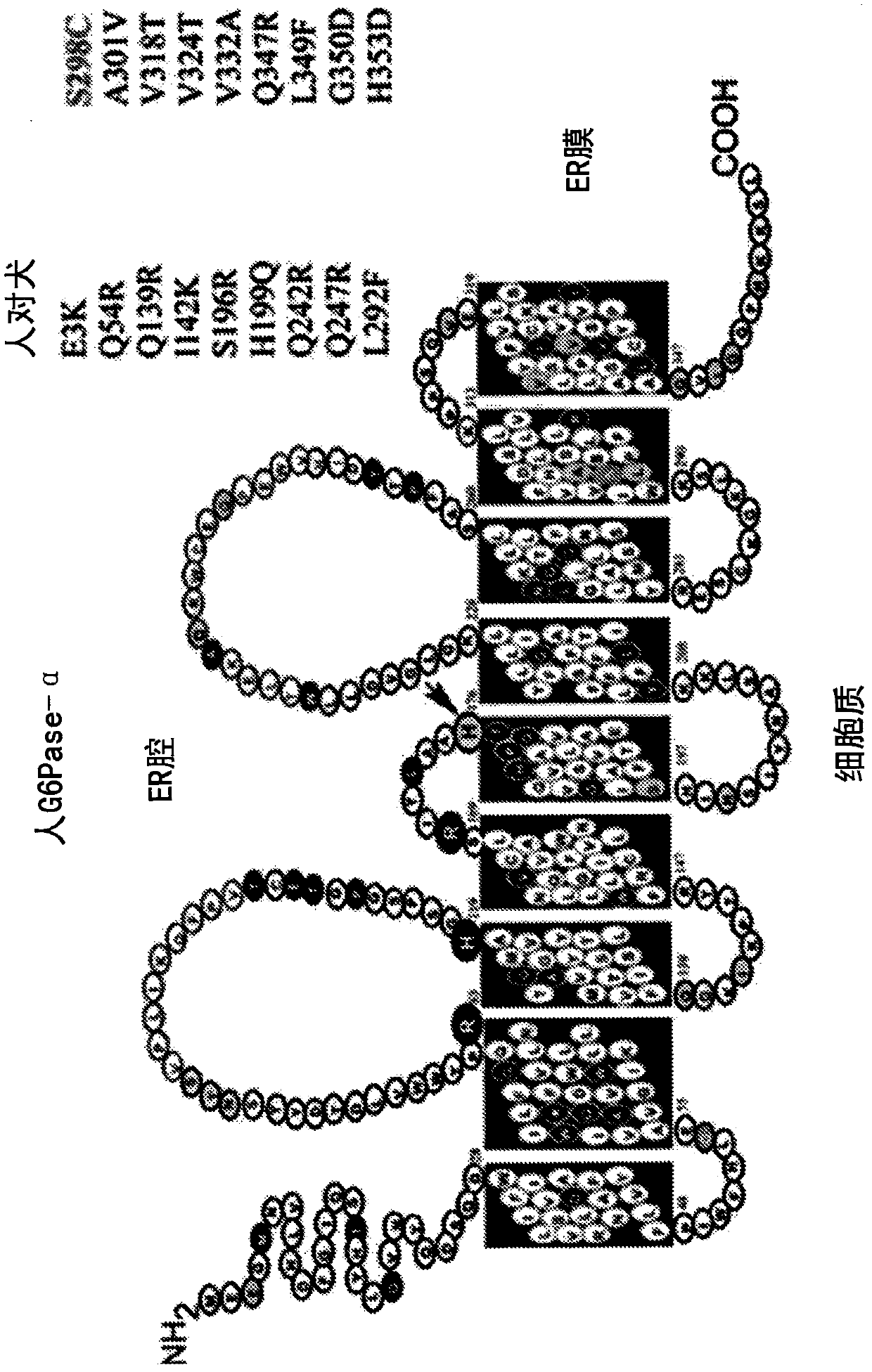
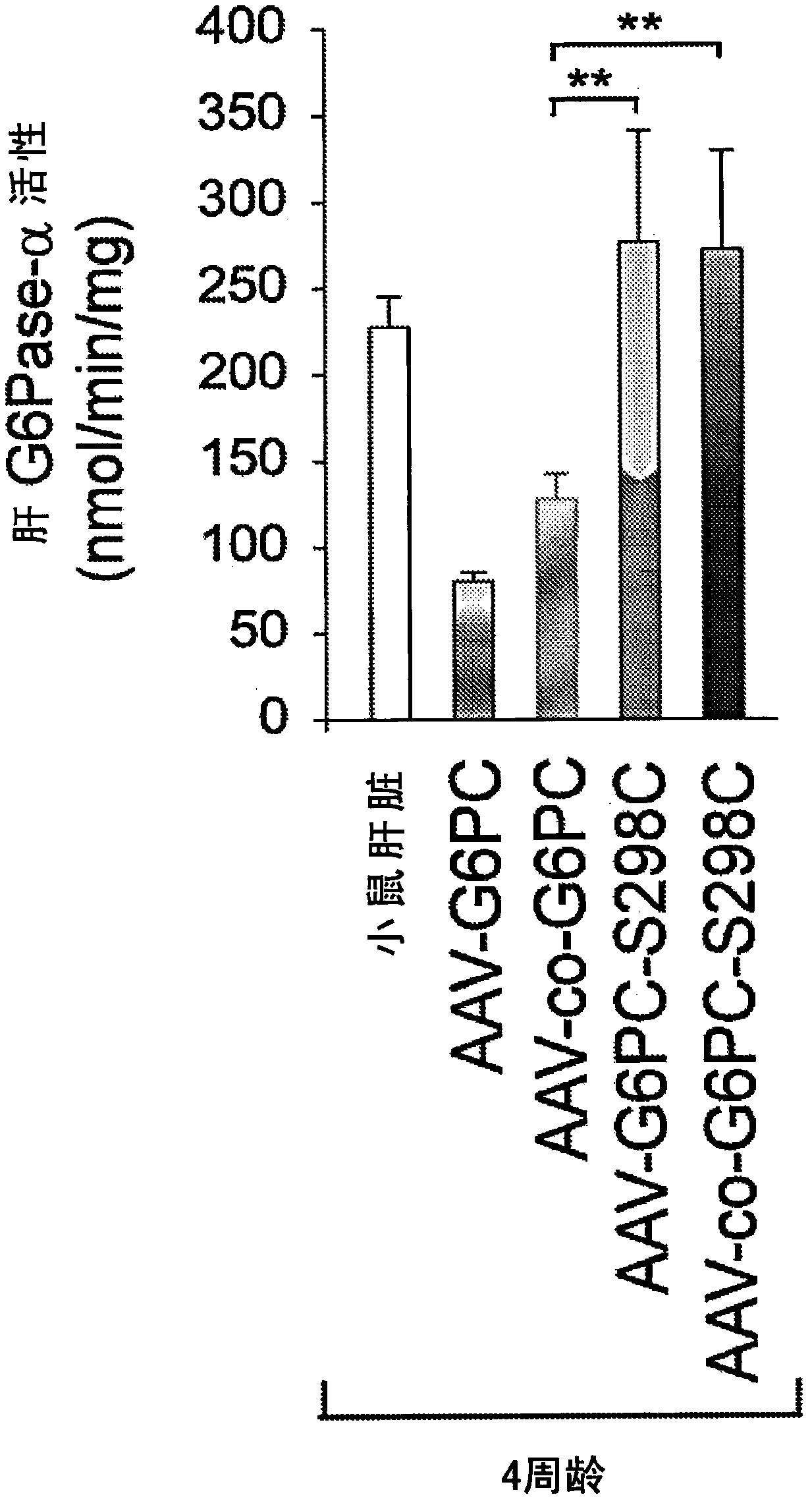
Modified G6PC (glucose-6-phosphatase, catalytic subunit) nucleic acids and glucose-6-phosphatase-alpha (G6Pase-alpha) enzymes with increased phosphohydrolase activity are described. Also described arevectors, such as adeno-associated virus (AAV) vectors, and recombinant AAV expressing modified G6Pase-alpha. The disclosed AAV vectors and rAAV can be used for gene therapy applications in the treatment of glycogen storage disease, particularly glycogen storage disease type la (GSD-Ia), and complications thereof.
Owner:UNITED STATES OF AMERICA
Methods and compositions for treatment of glycogen storage diseases and glycogen metabolism disorders
ActiveUS10202591B2Reduce deliveryReduce accumulationPolypeptide with localisation/targeting motifPeptide/protein ingredientsAlpha-amylaseGlycogen metabolism
Owner:VALERION THERAPEUTICS
Primer combinations, methods and kits for multiple genetic metabolic liver disease targeting libraries
ActiveCN109468372BSave time and costEasy to operateMicrobiological testing/measurementLibrary creationBile JuiceSuccinic acid
The invention discloses a primer combination, method and kit for constructing a targeted library of multiple inherited metabolic liver diseases based on high-throughput sequencing. The base sequence of the primer combination is shown as SEQ ID No. 1 to SEQ ID No. 2245, and the primer combination covers total 703 exons and cutting regions of 43 genes such as SERPINA1, G6PC, SLC37A4, AGL and the like, and is capable of detecting 41 sub-type gene variation loci of totally 20 inherited metabolic liver diseases comprising alpha-1-antitrypsin deficiency, glycogen storage disease, citrullinemia, aminosuccinuria, hemochromatosis, porphyrinopathy, cystic fibrosis, bile acid synthesis defect, Gaucher disease, hereditary fructose intolerance, cholesterol ester storage disease, Gilbert syndrome, Dubinsyndrome, Rotor syndrome, progressive familial intrahepatic choleatasia and the like. The primer combination disclosed by the invention is simple in operation, high in accuracy and low in time consumption, and the time cost and manual cost used for detecting genes and fragments one by one during Sanger sequencing can be greatly saved.
Owner:THE FIRST AFFILIATED HOSPITAL OF ARMY MEDICAL UNIV
New application of recombinant human thymosin alpha collagens
The invention discloses a new application of recombinant human thymosin alpha collagens, which relates to a recombinant human thymosin alpha collagen. The invention also provides the application of the recombinant human thymosin alpha collagen in preparing anti-fatigue drugs. The recombinant human thymosin alpha collagen (referred to as thymosin alpha collagen) refers to a recombinant human thymosin alpha collagen and a tissue separated and purified thymosin alpha collagen. The effect of the thymosin alpha collagen in various anti-fatigues is implemented through increasing the glycogen storage of the liver and muscles and reducing the level of serum urea nitrogen so as to improve the fatigue resistance.
Owner:XIAMEN UNIV
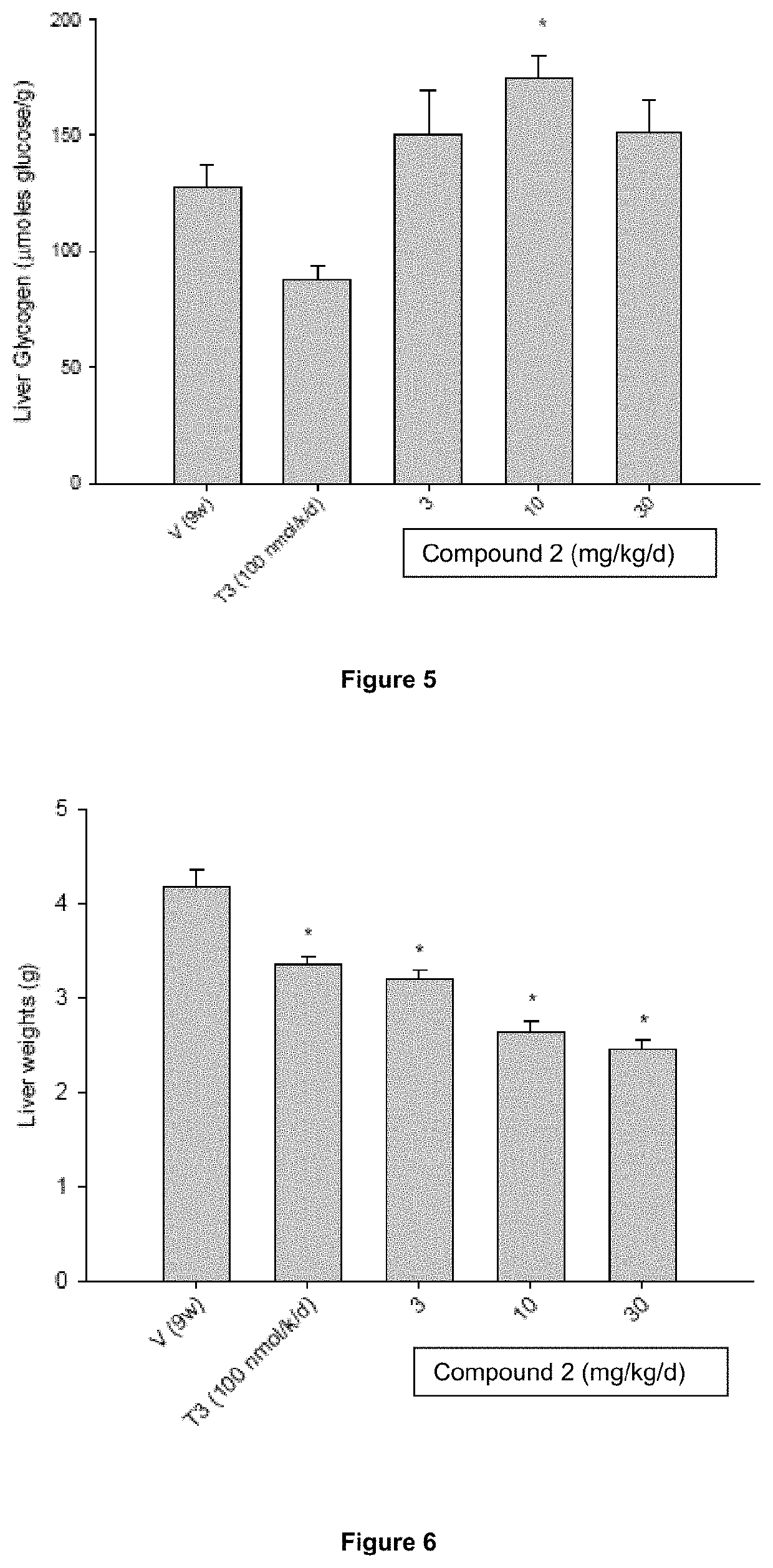
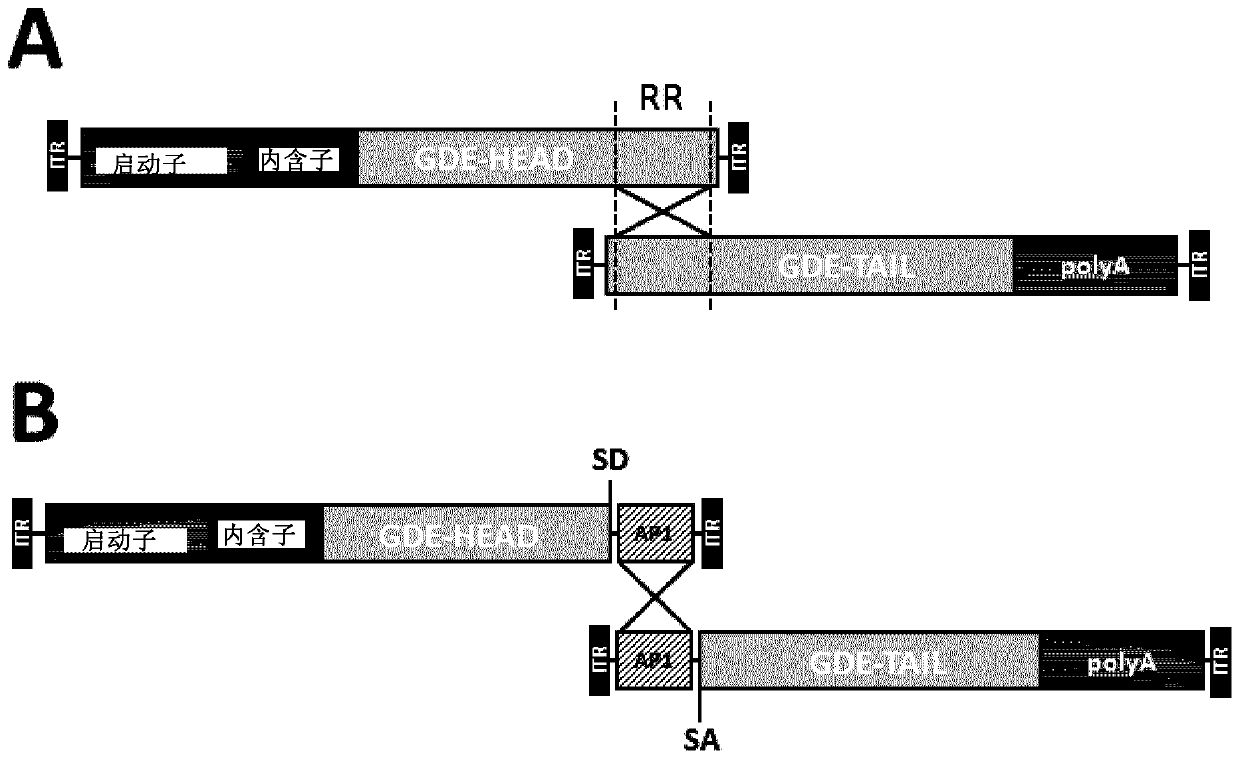
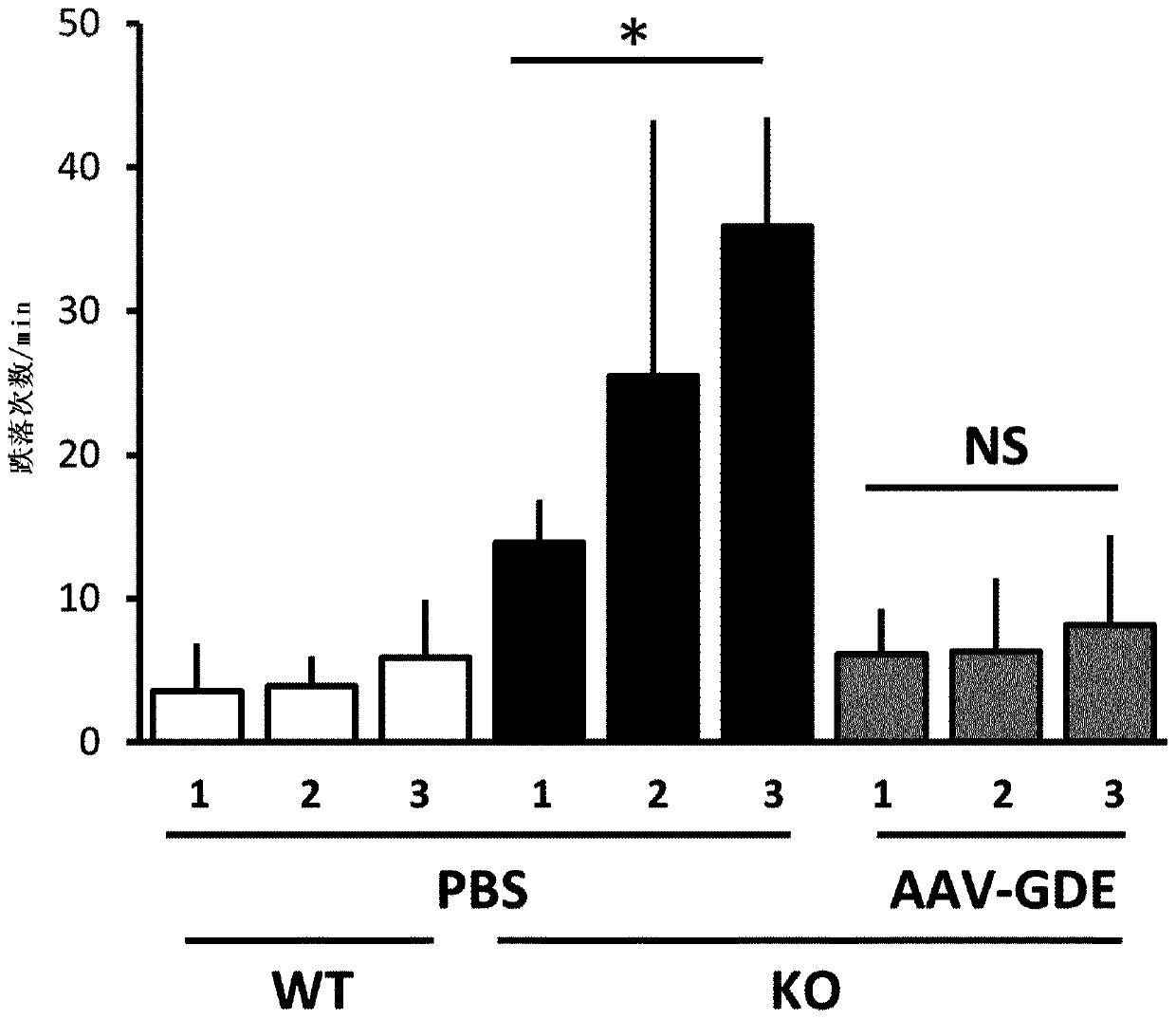
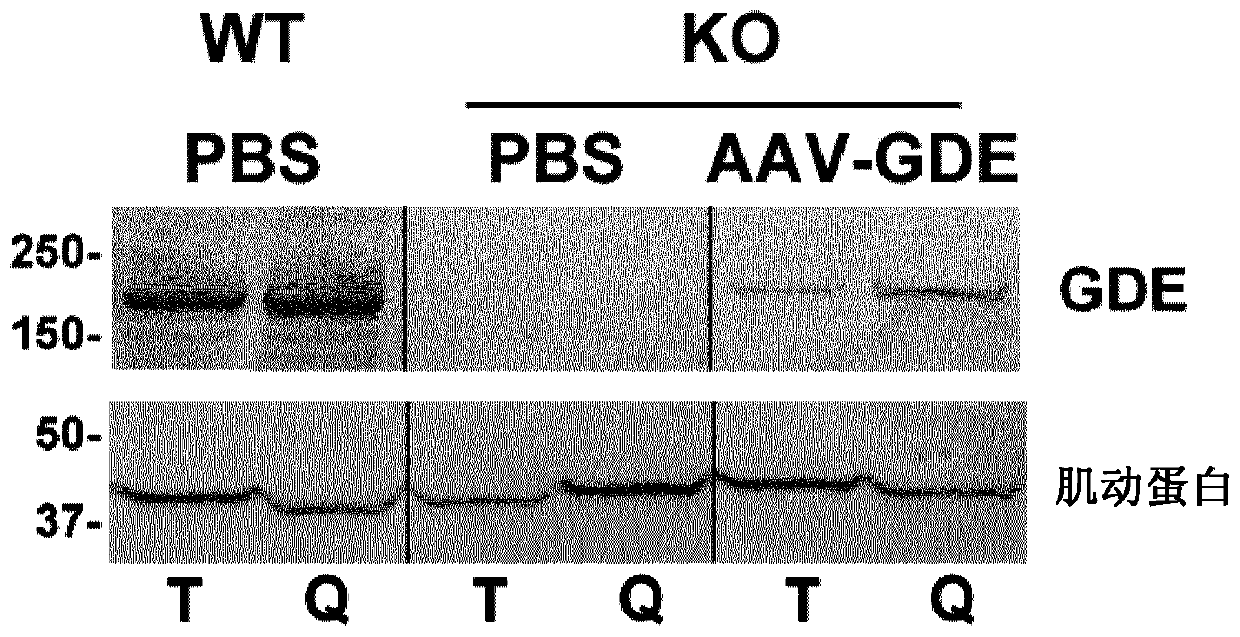
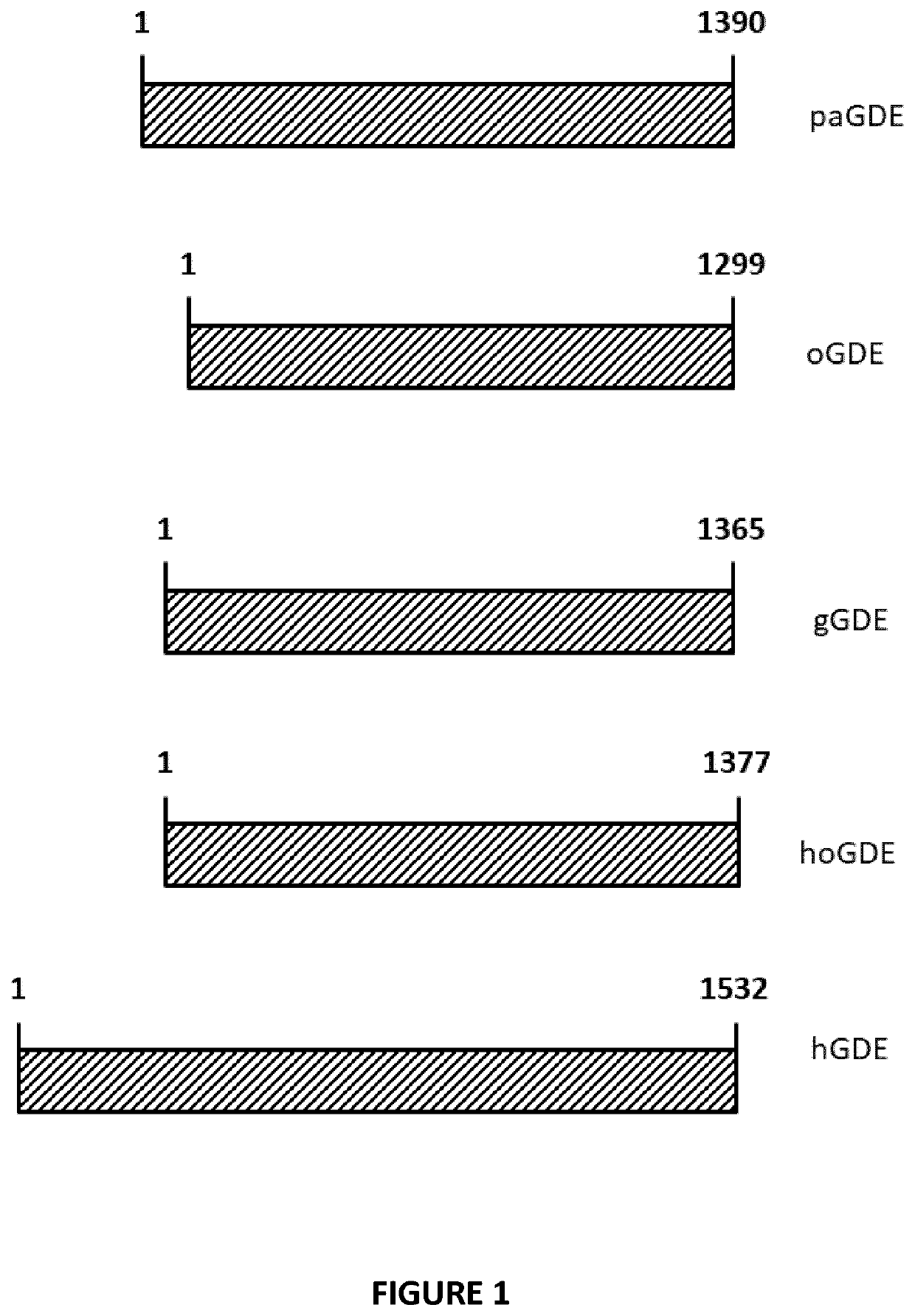
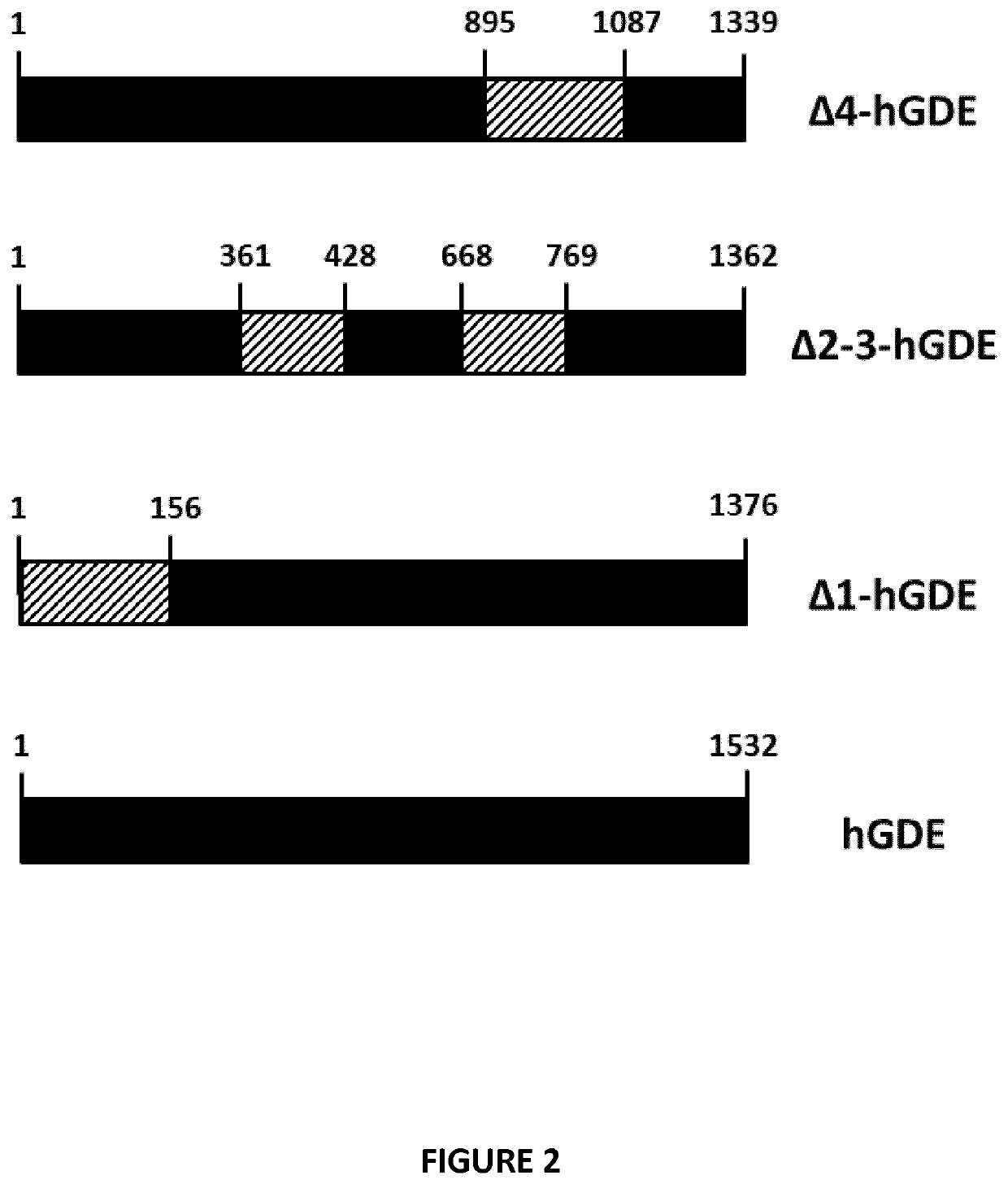
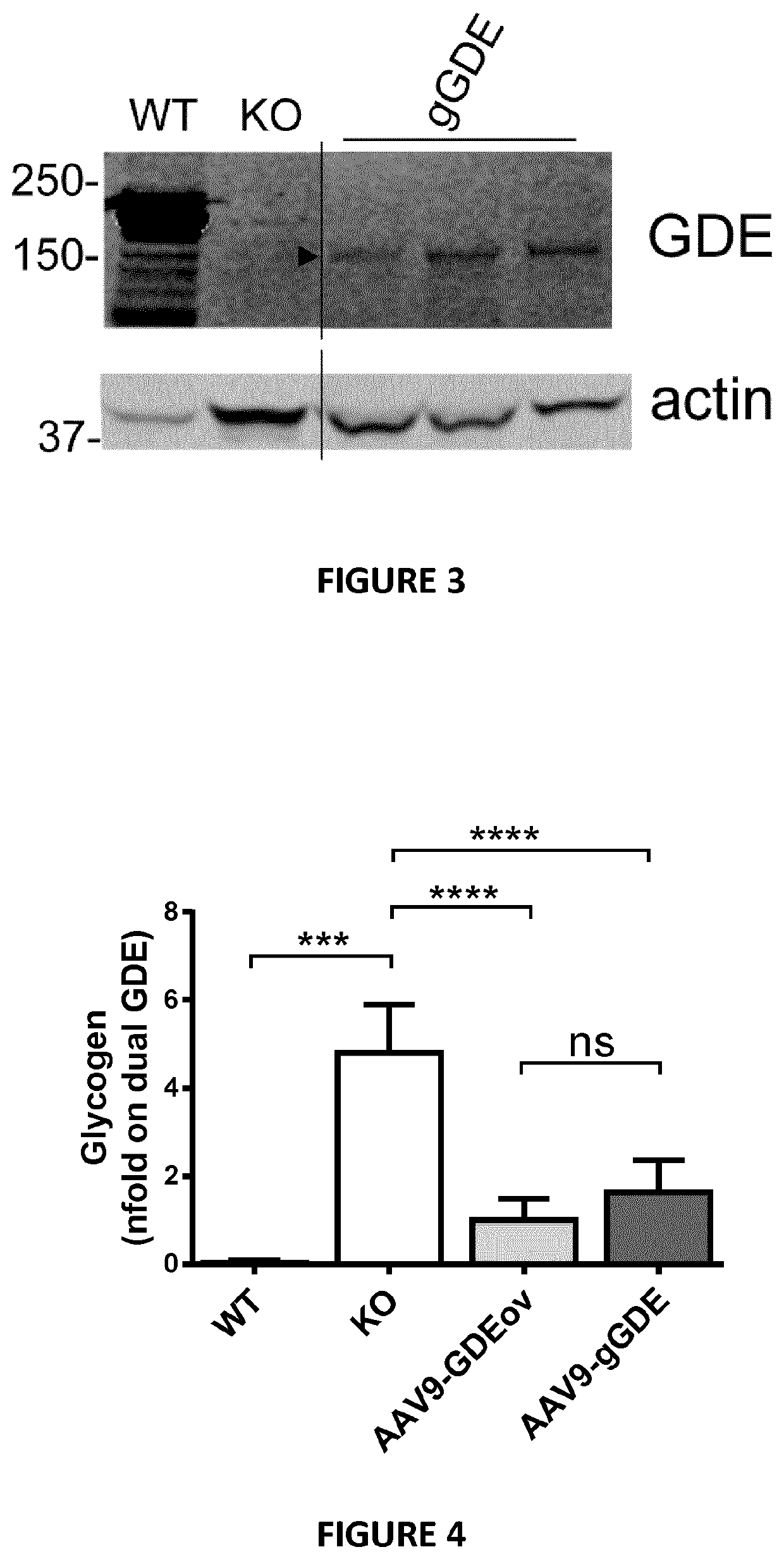
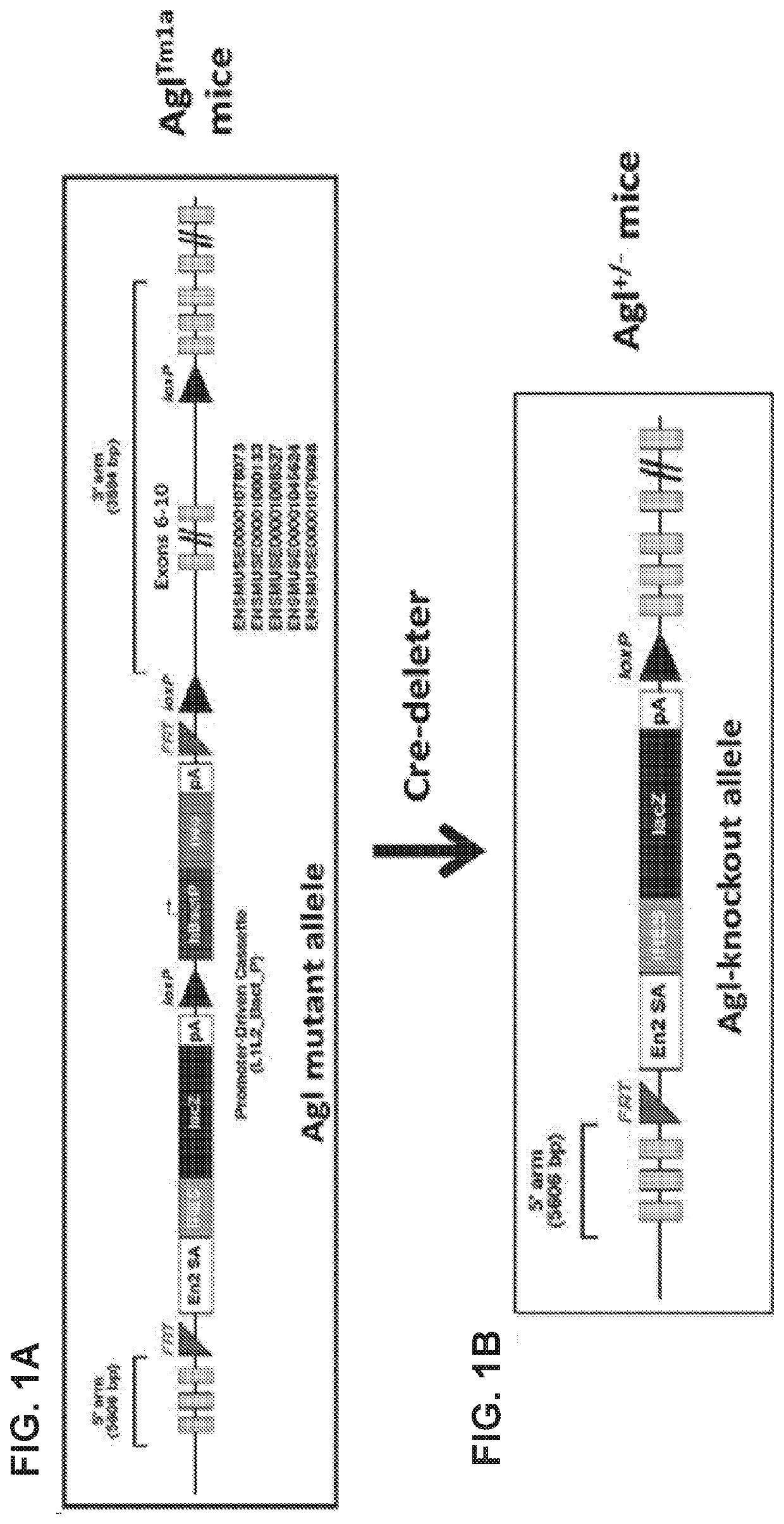
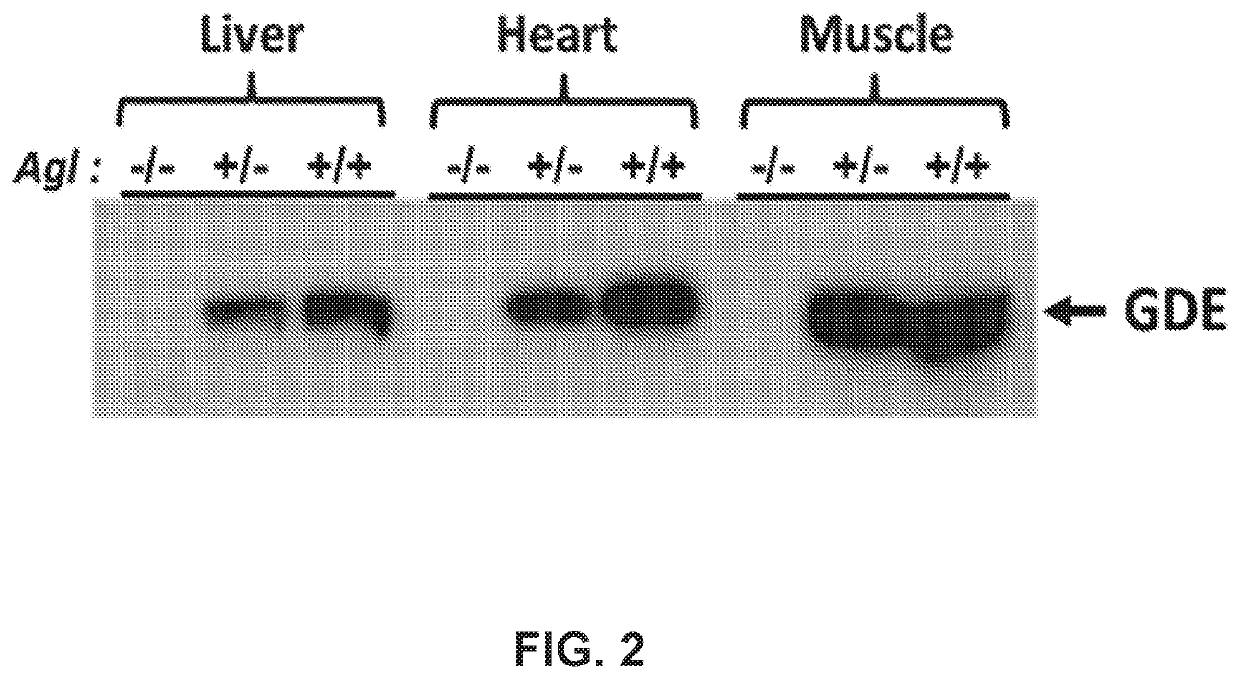
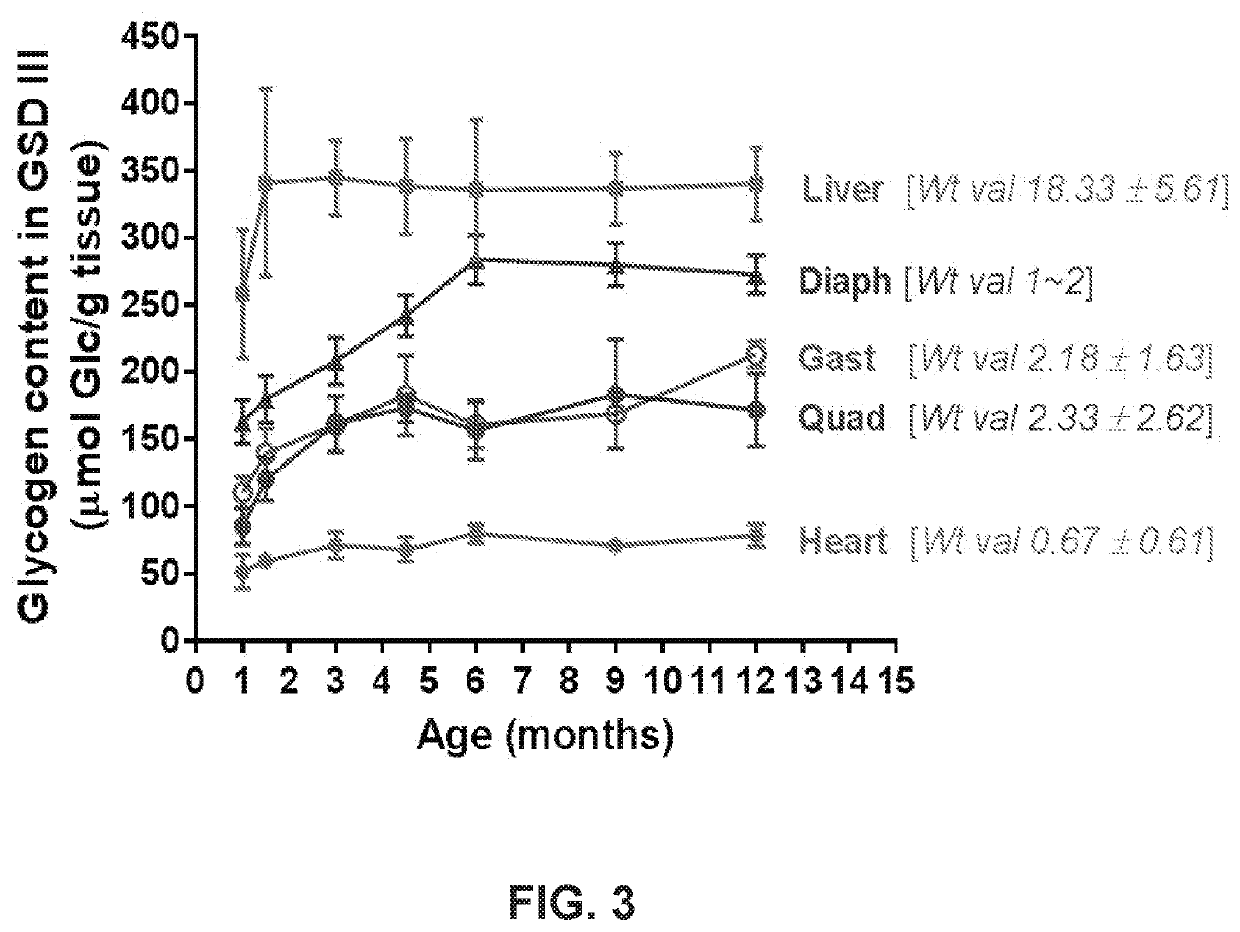
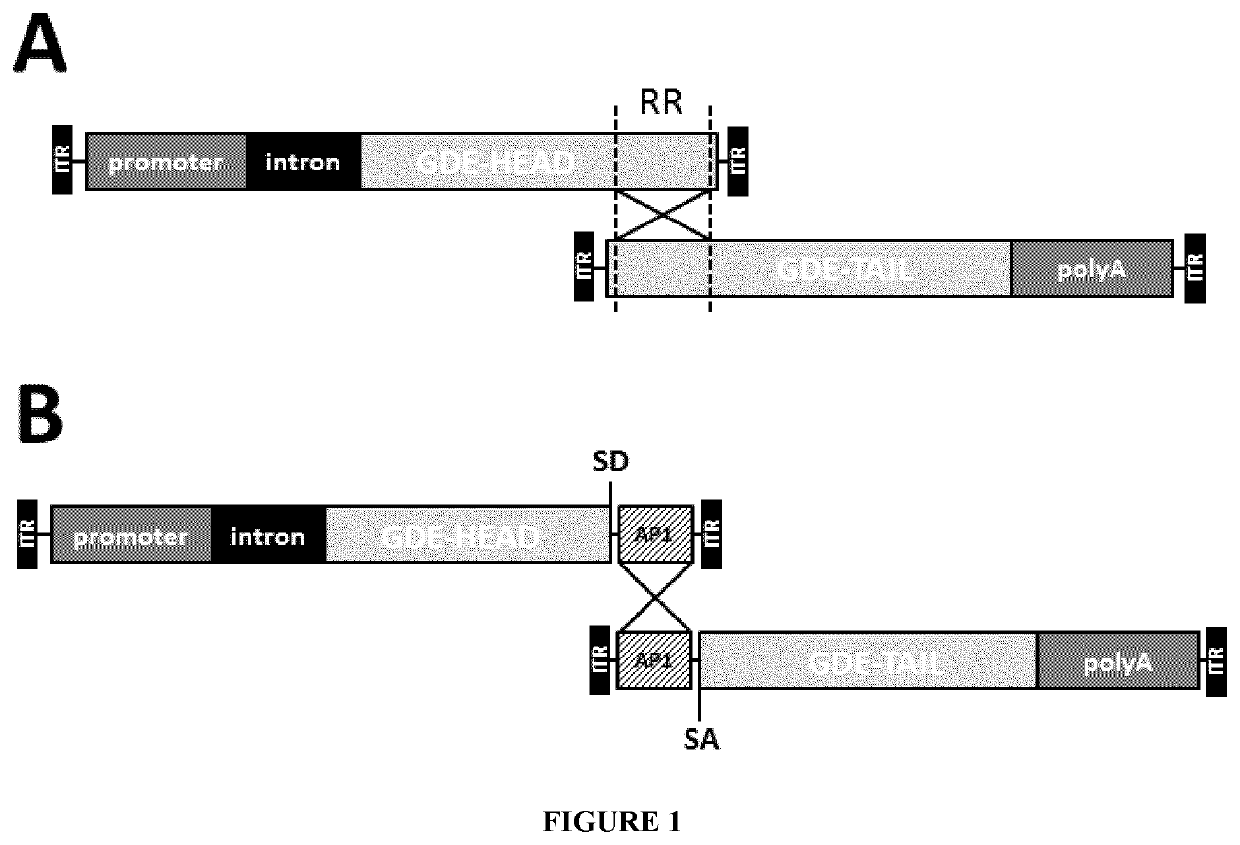
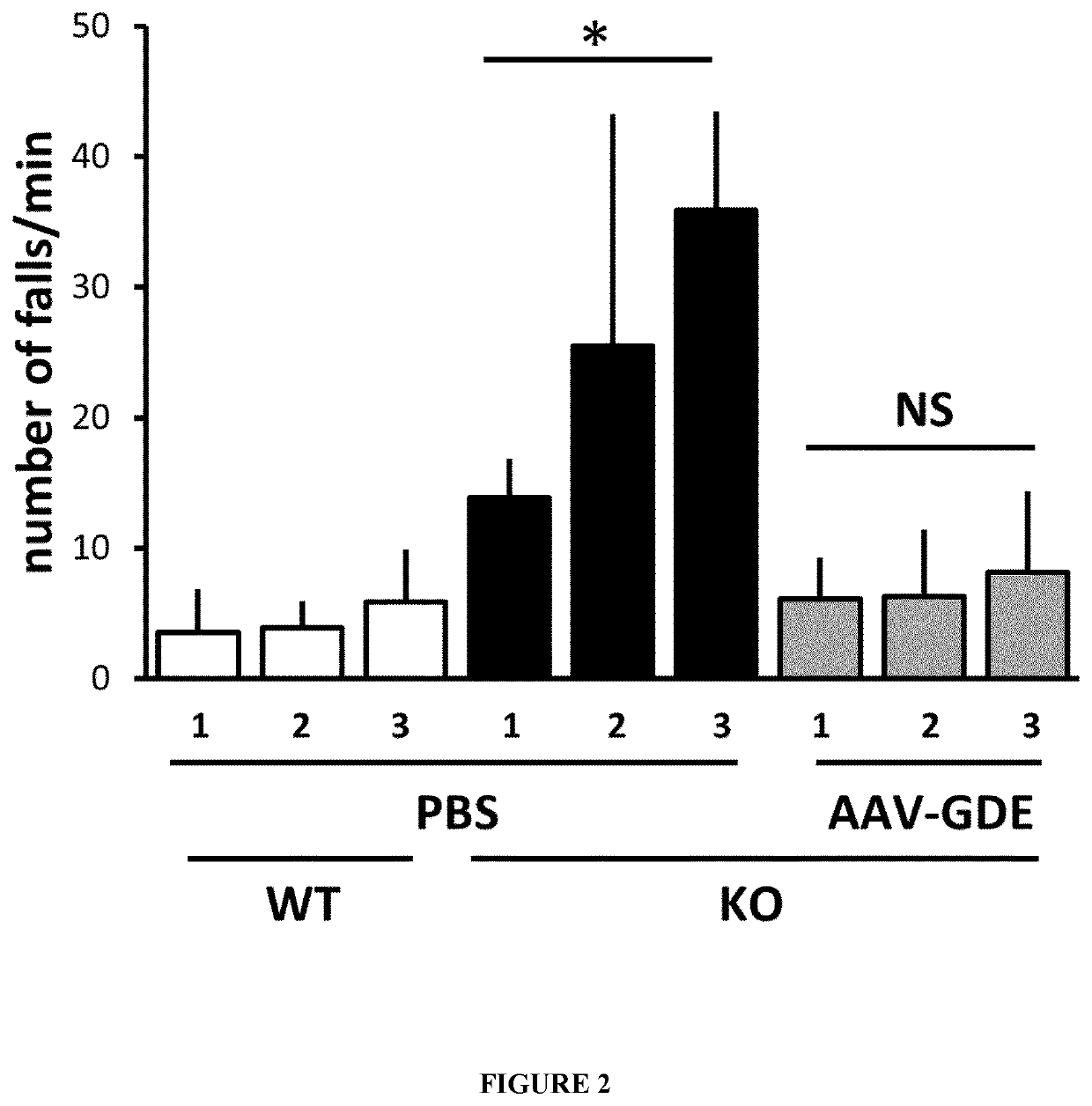
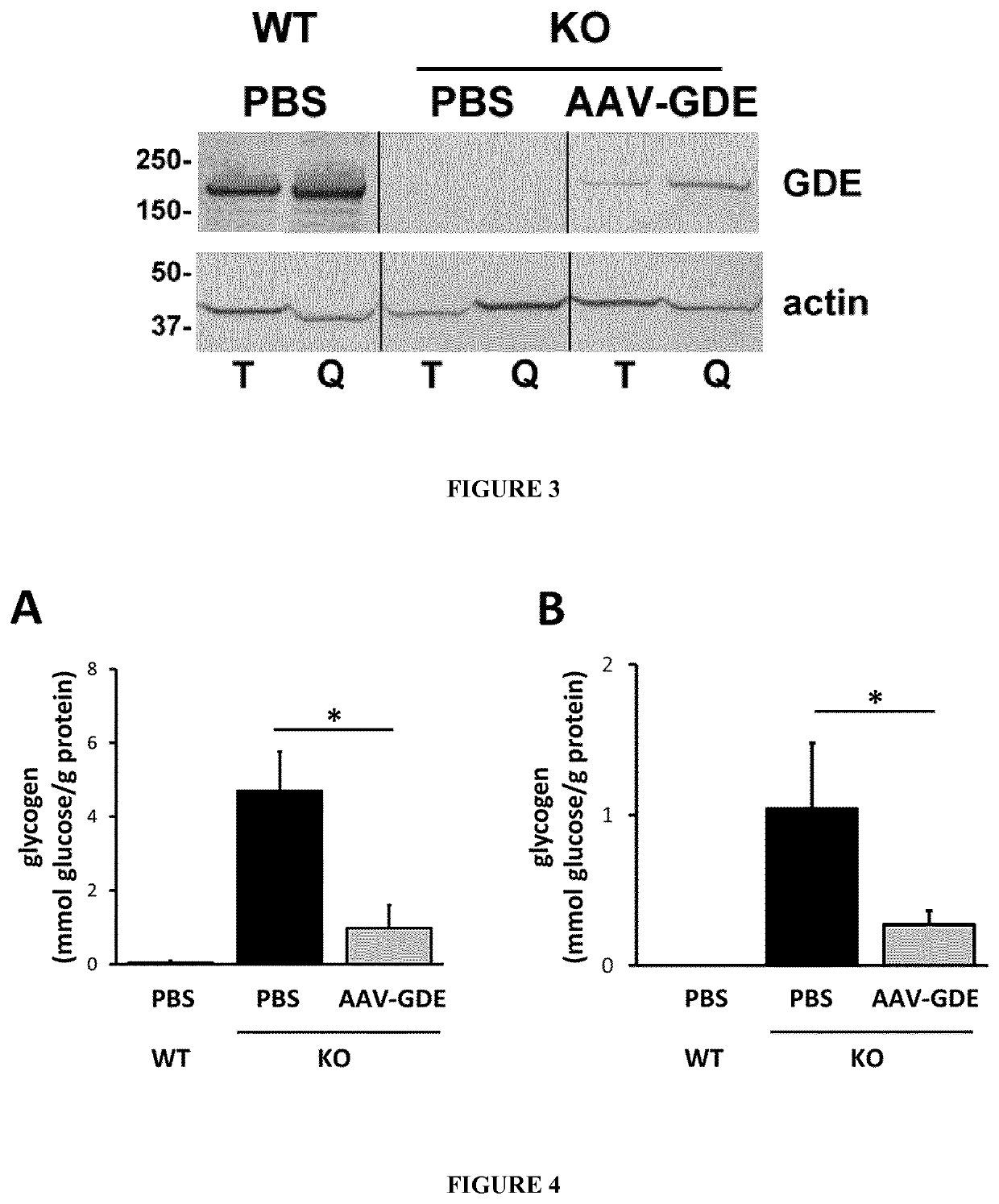
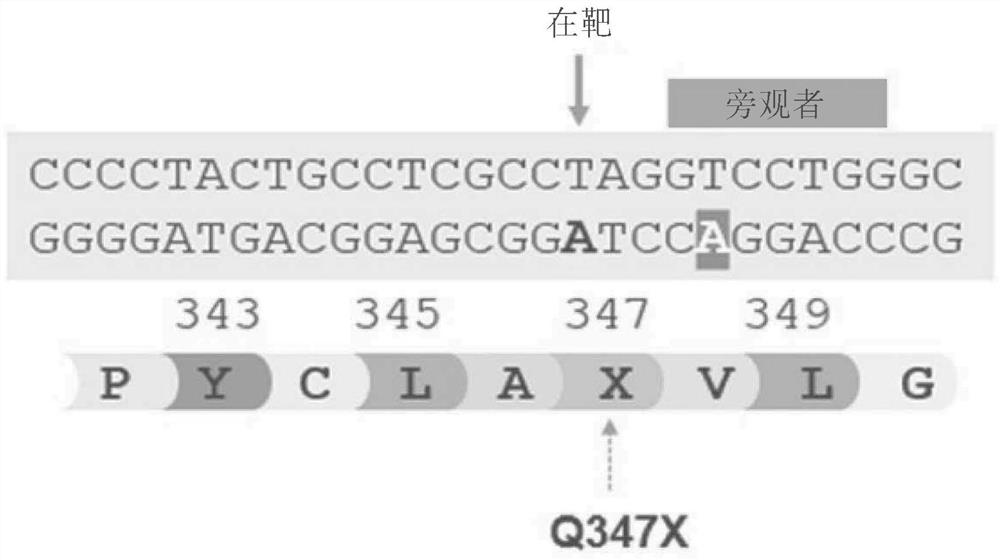
Mini-gde for the treatment of glycogen storage disease iii
Owner:GENETHON +4
Deleted adenovirus vectors and methods of making and administering the same
The present invention provides deleted adenovirus vectors. The inventive adenovirus vectors carry one or more deletions in the IVa2, 100 K, polymerase and / or preterminal protein sequences of the adenovirus genome. The adenoviruses may additionally contain other deletions, mutations or other modifications as well. In particular preferred embodiments, the adenovirus genome is multiply deleted, i.e., carries two or more deletions therein. The deleted adenoviruses of the invention are “propagation-defective” in that the virus cannot replicate and produce new virions in the absence of complementing function(s). Preferred adenovirus vectors of the invention carry a heterologous nucleotide sequence encoding a protein or peptide associated with a metabolic disorder, more preferably a protein or peptide associated with a lysosomal or glycogen storage disease, most preferably, a lysosomal acid α-glucosidase. Further provided are methods for producing the inventive deleted adenovirus vectors. Further provided are methods of administering the deleted adenovirus vectors to a cell in vitro or in vivo.
Owner:DUKE UNIV
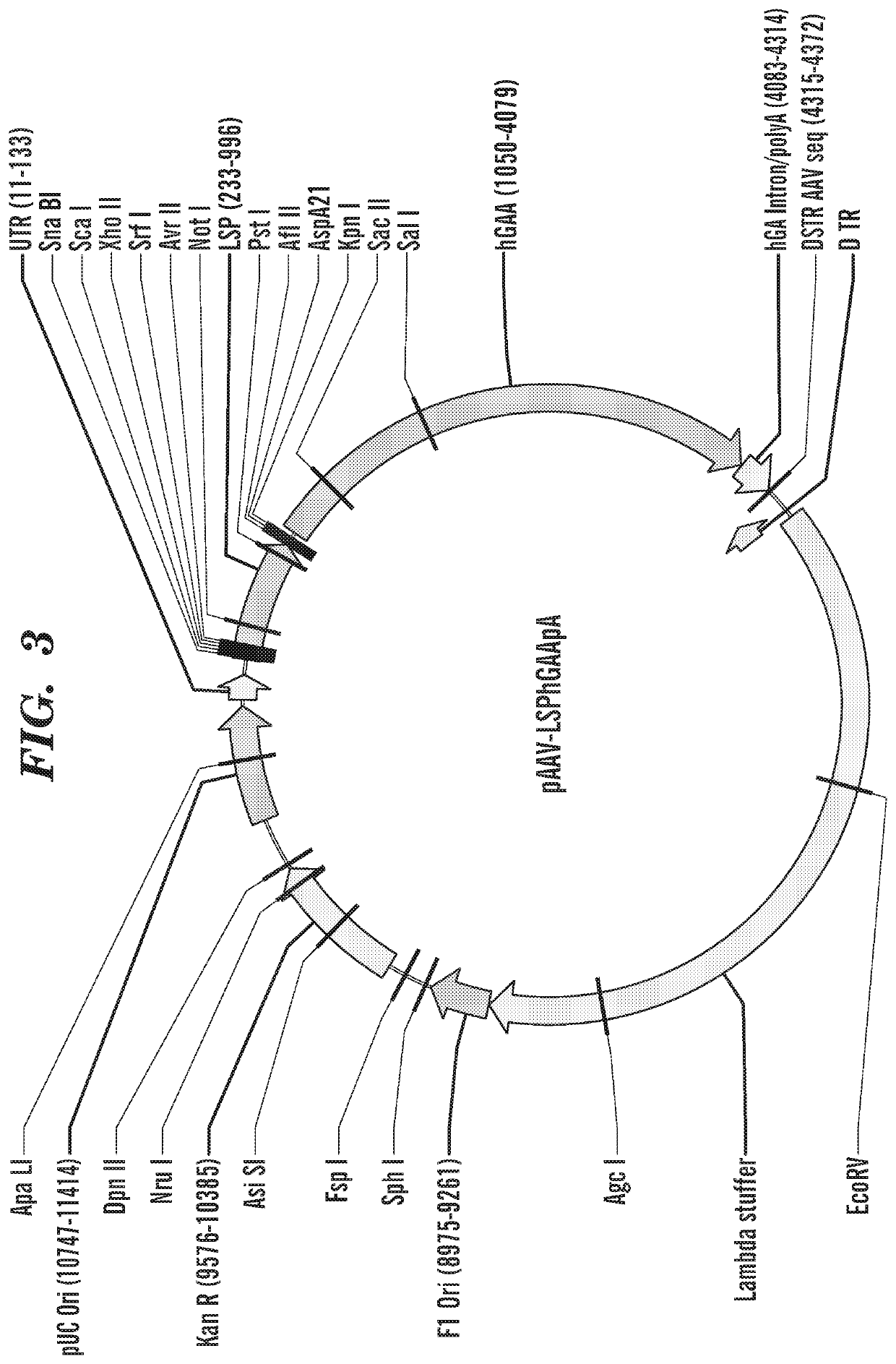
Methods and compositions for treating glycogen storage diseases
This invention provides a variety of novel adeno-associated virus (AAV) vectors for gene therapy applications in the treatment of glycogen storage disease type la (GSD-Ia). Disclosed herein are a number of recombinant nucleic acid molecules, vectors and recombinant AAV that incorporate a modified G6PC promoter / enhancer sequence. Utilization of the modified G6PC promoter / enhancer sequence results in enhanced AAV yield and quality when expressed from various host cell platforms. Also provided herein are compositions comprising the novel AAV of the invention and methods of treating GSD-Ia using the same.
Owner:阿尔特拉吉尼克斯制药公司
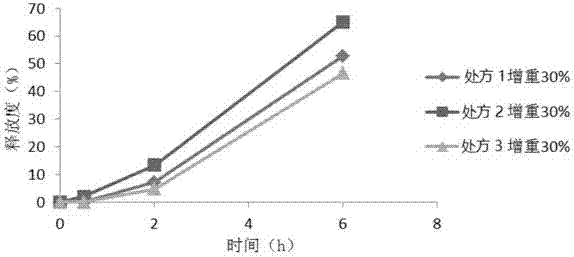
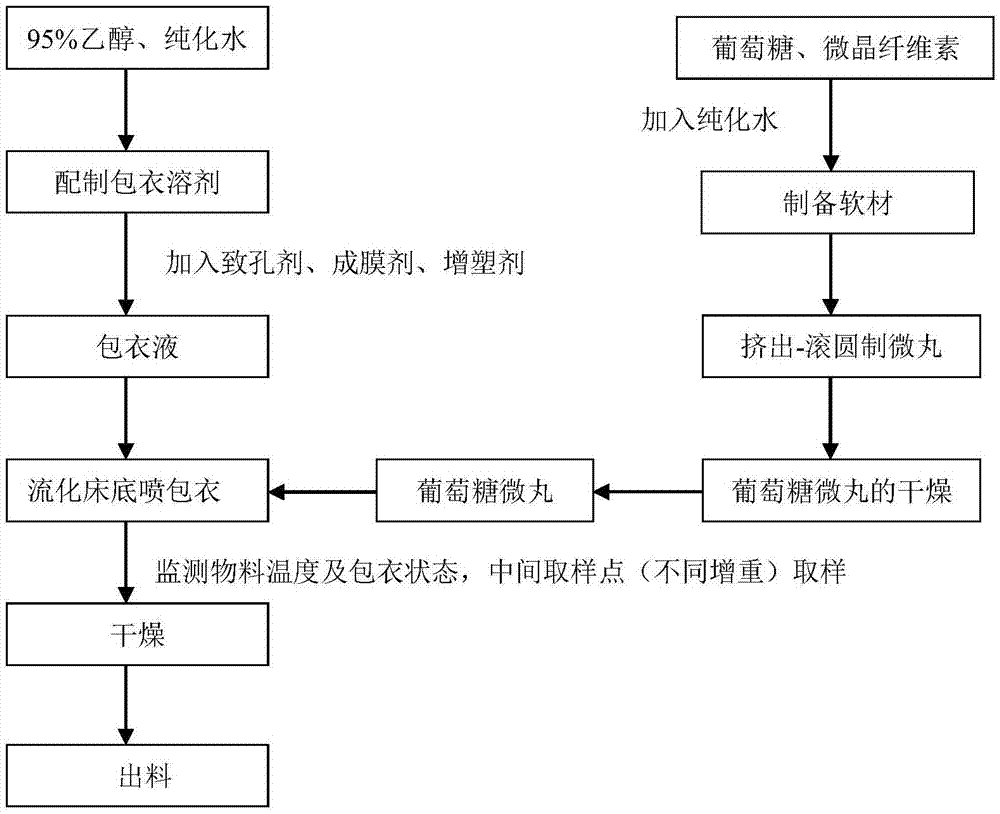
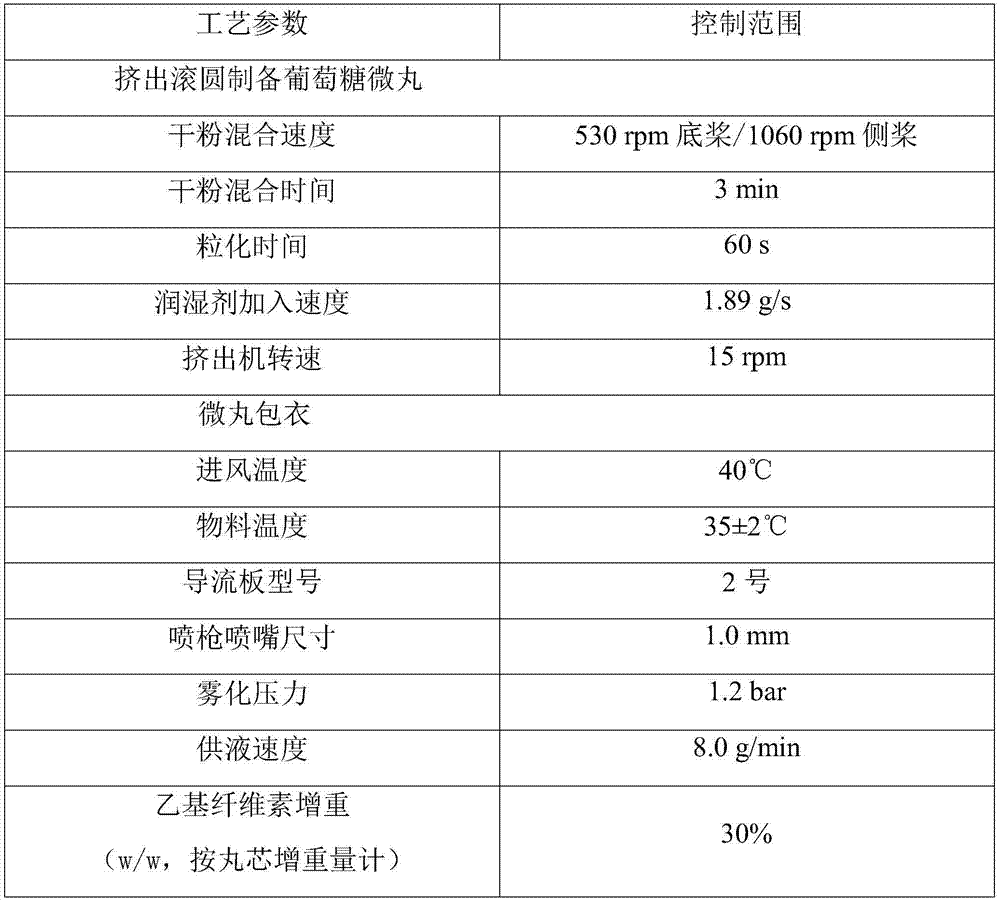
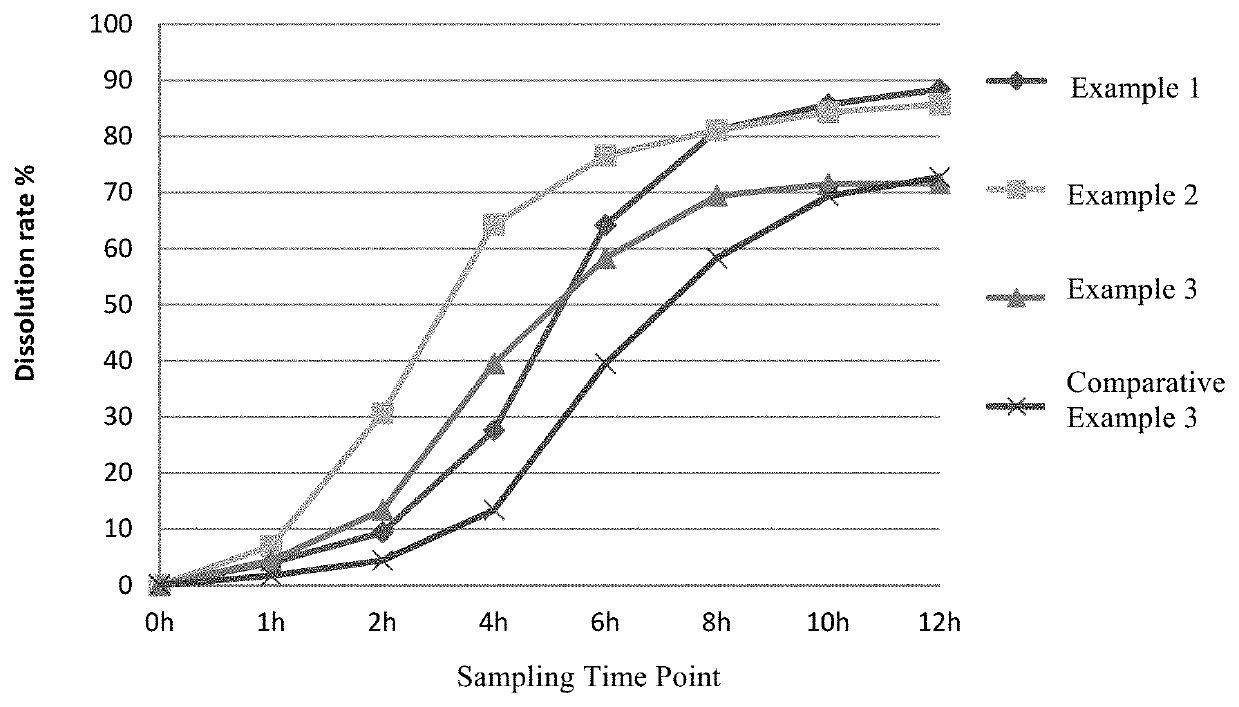
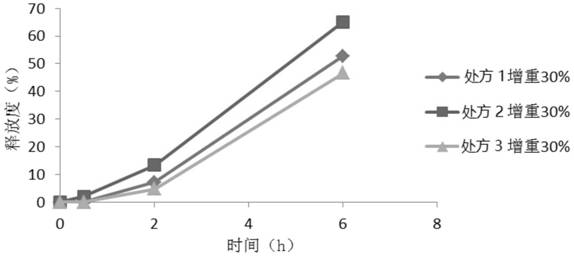
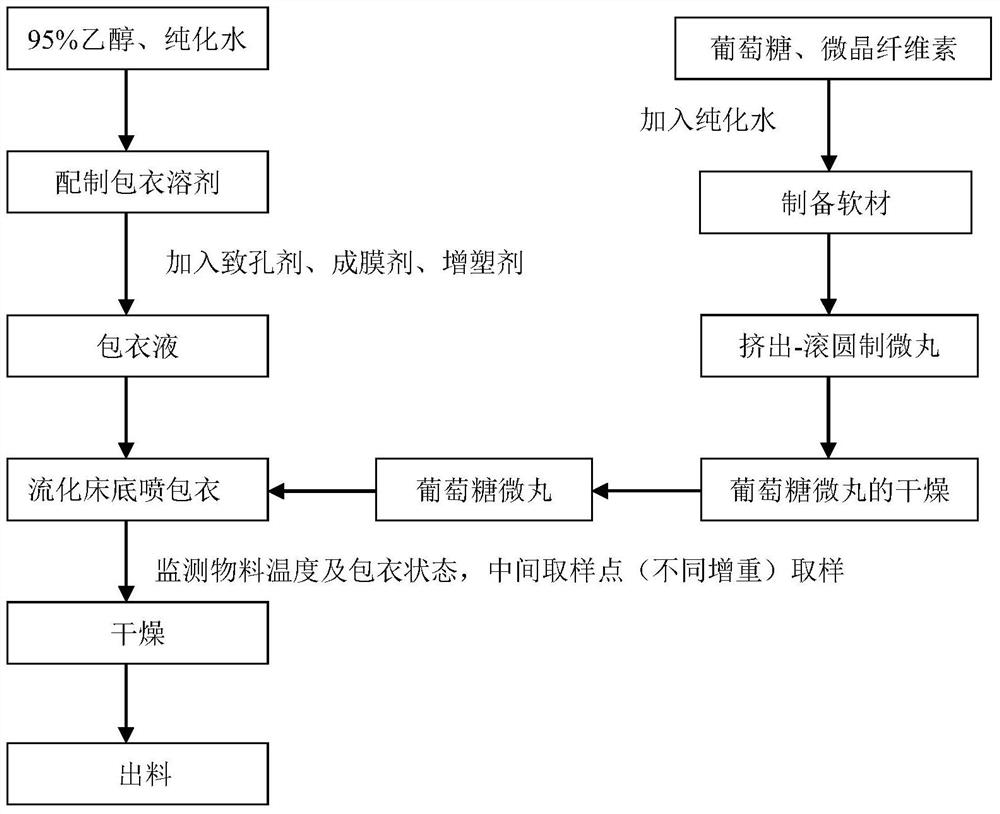
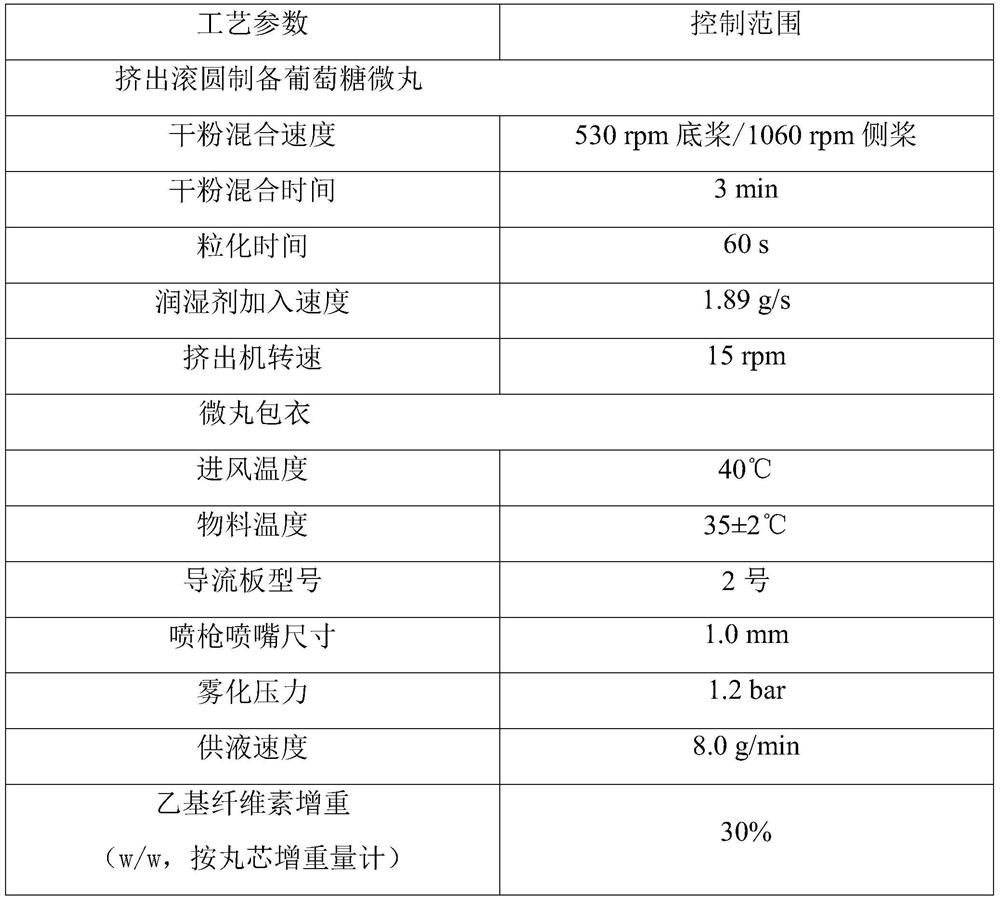
Sustained-release glucose preparation for treating glycogen storage diseases
ActiveCN106913558AGood slow releaseOrganic active ingredientsMetabolism disorderPharmaceutical formulationFilm coating
The invention provides a sustained-release glucose preparation for treating glycogen storage diseases and a preparation method thereof, belonging to the field of medicinal preparations. The sustained-release glucose preparation is composed of two parts, i.e., a glucose pill core and a film coating and prepared from glucose monohydrate, microcrystalline cellulose, ethyl cellulose, hydroxypropyl cellulose, triethyl citrate, ethanol and purified water by using a modern preparation technology. Results of release-rate determination experiments prove that the sustained-release glucose preparation provided by the invention has good sustained-release performance and can be gradually and slowly released within 6 h; the release rates of sustained-release glucose preparations prepared according to three different prescriptions are 40 to 70% within 6 h; so the sustained-release glucose preparation can replace raw core starch and be used for treatment of glycogen storage diseases.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI +2
Glucose pellets, preparation method and use thereof
The invention belongs to the field of biomedicine, and particularly relates to a glucose core, comprising a glucose and / or a glucose hydrate, a diluent and a binder, wherein, the glucose and / or the glucose hydrate is present in the glucose core in an amount of ≤85% by weight percentage; and relates to a glucose pellet comprising the glucose core and a laminated layer coating the glucose core; and further relates to a pharmaceutical composition comprising the glucose pellet. The glucose pellet or the pharmaceutical composition of the invention are useful in treatment and / or assistant treatment of a glycogen storage disease and / or a diabetes.
Owner:COSCI MED TECH CO LTD
Compositions and Methods for the Treatment of Genetic Diseases
PendingUS20220105204A1Peptide/protein ingredientsGenetic material ingredientsDual promoterNucleic acid sequencing
This disclosure provides isolated nucleic acid molecules comprising nucleic acid sequences encoding microbial polypeptides that are codon optimized for expression in mammalian cells, vectors comprising an immunotolerant dual promoter system, and methods using these polynucleotides and polypeptides to treat glycogen storage diseases and other inherited diseases.
Owner:DUKE UNIV
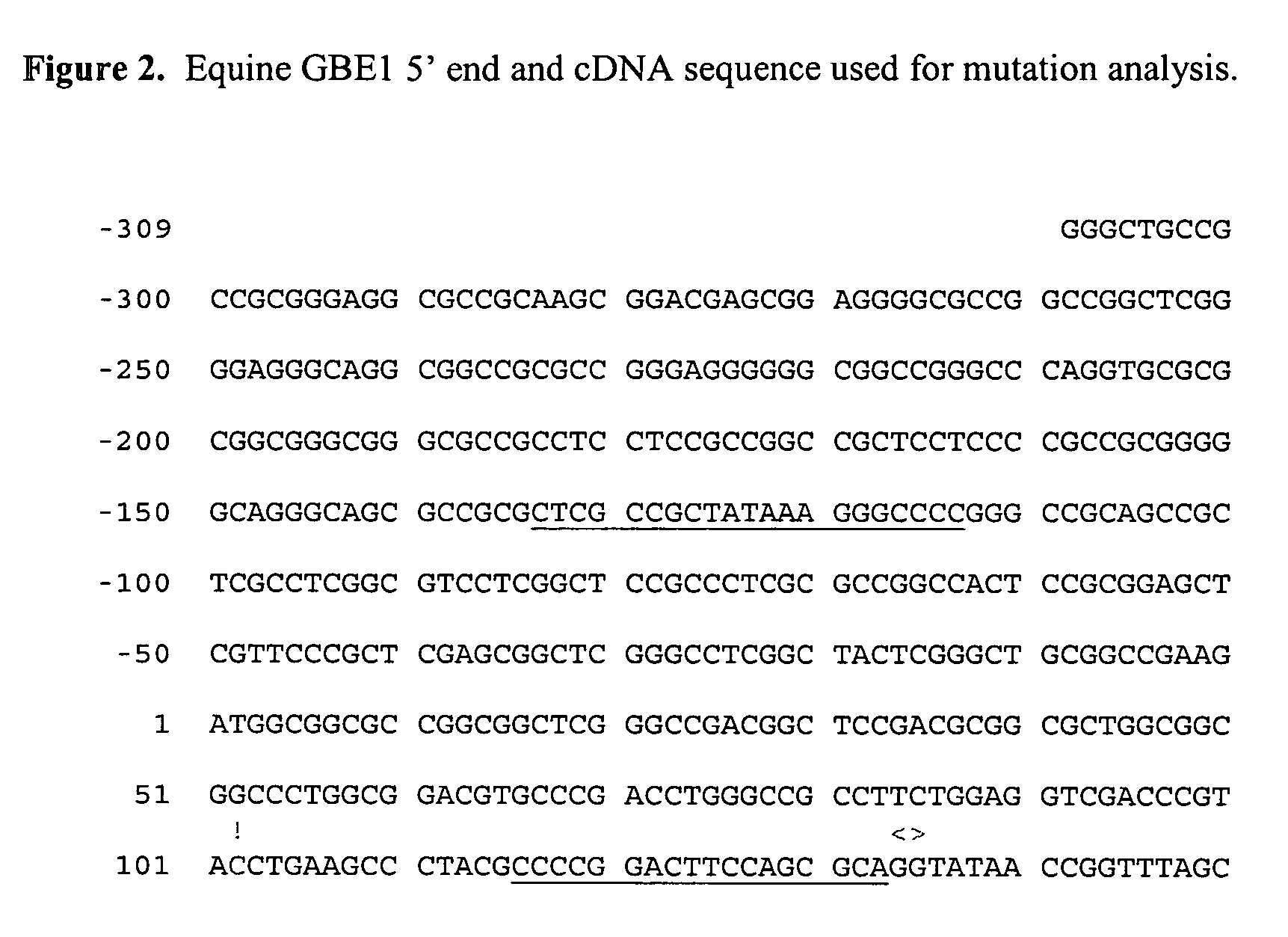
Method of detecting equine glycogen storage disease IV
Owner:RGT UNIV OF MINNESOTA
Treatment of glycogen storage disease iii
ActiveUS20200289673A1Decrease muscle glycogen accumulationHydrolasesMetabolism disorderMolecular biologyGlycogen storage disease
Owner:GENETHON +3
Compositions and methods for treating glycogen storage disease type 1a
PendingCN114026237AOrganic active ingredientsFusion with DNA-binding domainMolecular biologyGlycogen storage disease
Owner:BEAM THERAPEUTICS INC
A glucose sustained-release preparation for treating glycogen accumulation disease
ActiveCN106913558BGood slow releaseOrganic active ingredientsMetabolism disorderPharmaceutical formulationSustained-Release Preparations
The invention provides a sustained-release glucose preparation for treating glycogen storage diseases and a preparation method thereof, belonging to the field of medicinal preparations. The sustained-release glucose preparation is composed of two parts, i.e., a glucose pill core and a film coating and prepared from glucose monohydrate, microcrystalline cellulose, ethyl cellulose, hydroxypropyl cellulose, triethyl citrate, ethanol and purified water by using a modern preparation technology. Results of release-rate determination experiments prove that the sustained-release glucose preparation provided by the invention has good sustained-release performance and can be gradually and slowly released within 6 h; the release rates of sustained-release glucose preparations prepared according to three different prescriptions are 40 to 70% within 6 h; so the sustained-release glucose preparation can replace raw core starch and be used for treatment of glycogen storage diseases.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI +2
Vectors comprising a nucleic acid encoding lysosomal enzymes fused to a lysosomal teargeting sequence
PendingUS20220133906A1Polypeptide with localisation/targeting motifInsulin-like growth factorsHeterologousLysosomal targeting
Vectors including viral vectors comprising a genome comprising a heterologous nucleic acid encoding a lysosomal targeting sequence, fused to a lysosomal storage enzyme, enabling the lysosomal enzyme to be targeted to the lysosomes. Particular embodiments relate to a recombinant viral vector, e.g., rAAV vector encoding a lysosomal enzyme, having a lysosomal targeting IGF2(V43) sequence that binds human cation-independent mannose-6-phosphate receptor (CI-MPR) or to the IGF2 receptor, permitting proper subcellular localization of the lysosomal enzyme polypeptide to lysosomes. Also encompassed are therapeutic fusion proteins encoded by the viral vector, non-viral vectors, cells, and methods to treat a glycogen storage disease, e.g., those listed in Table 4A or Table 5A with the viral vector. t,?
Owner:ASKLEPIOS BIOPHARMACEUTICAL INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com