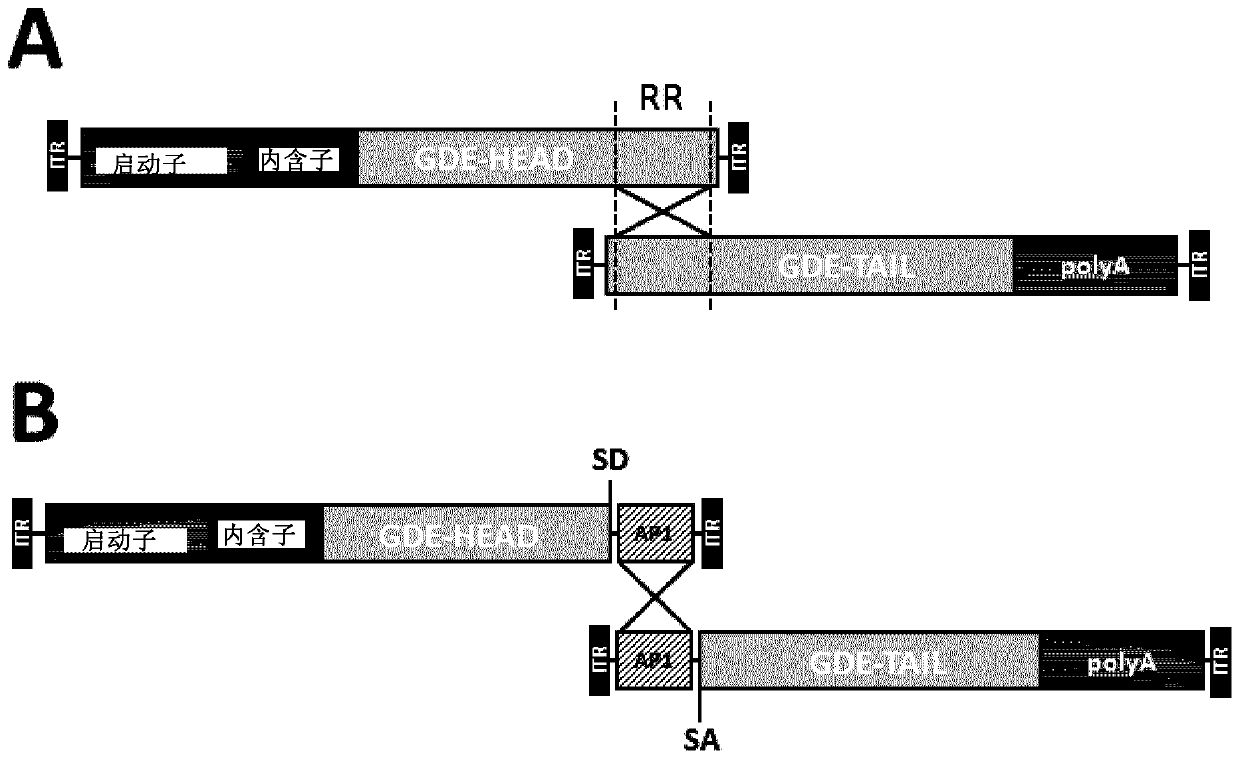
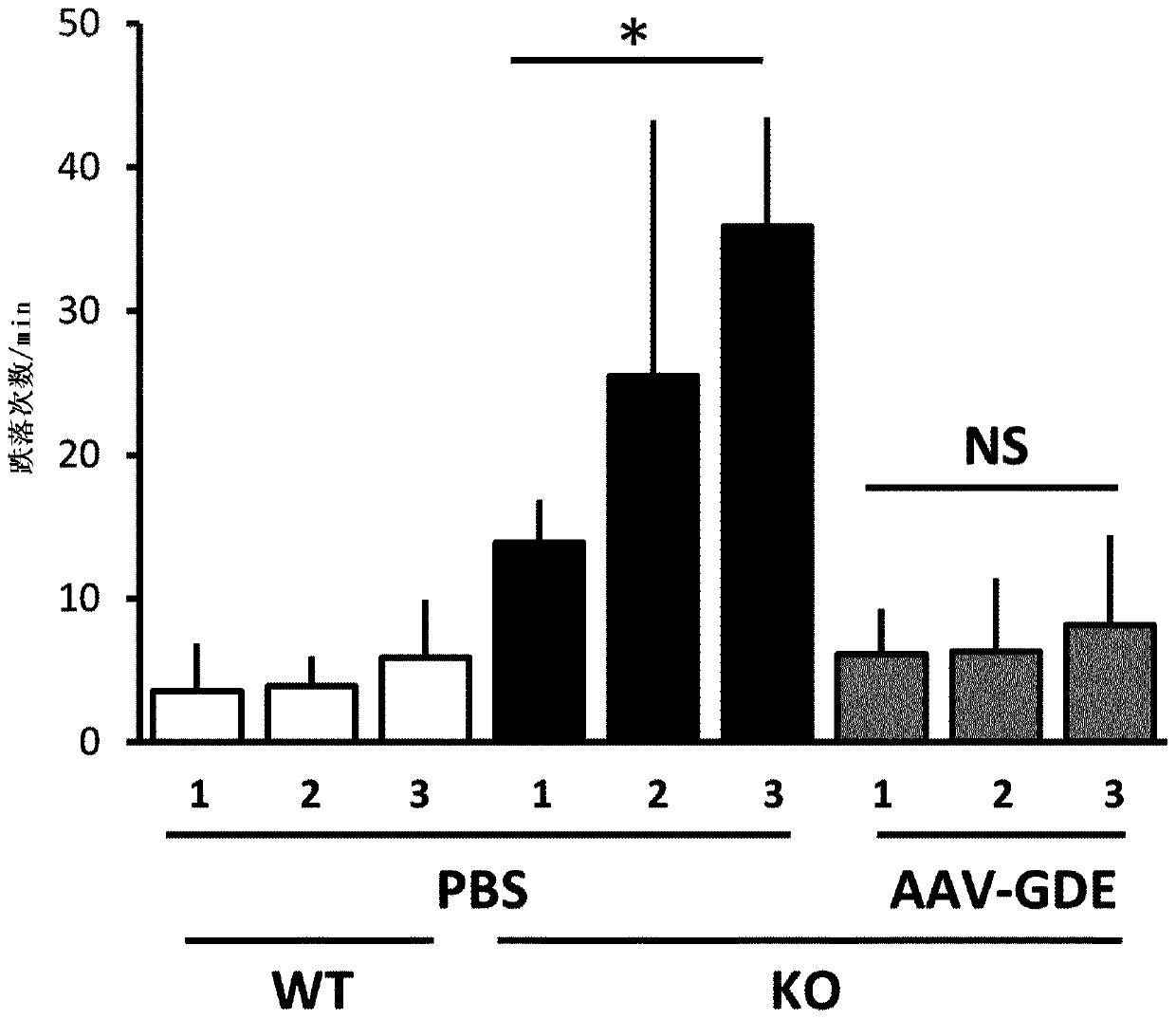
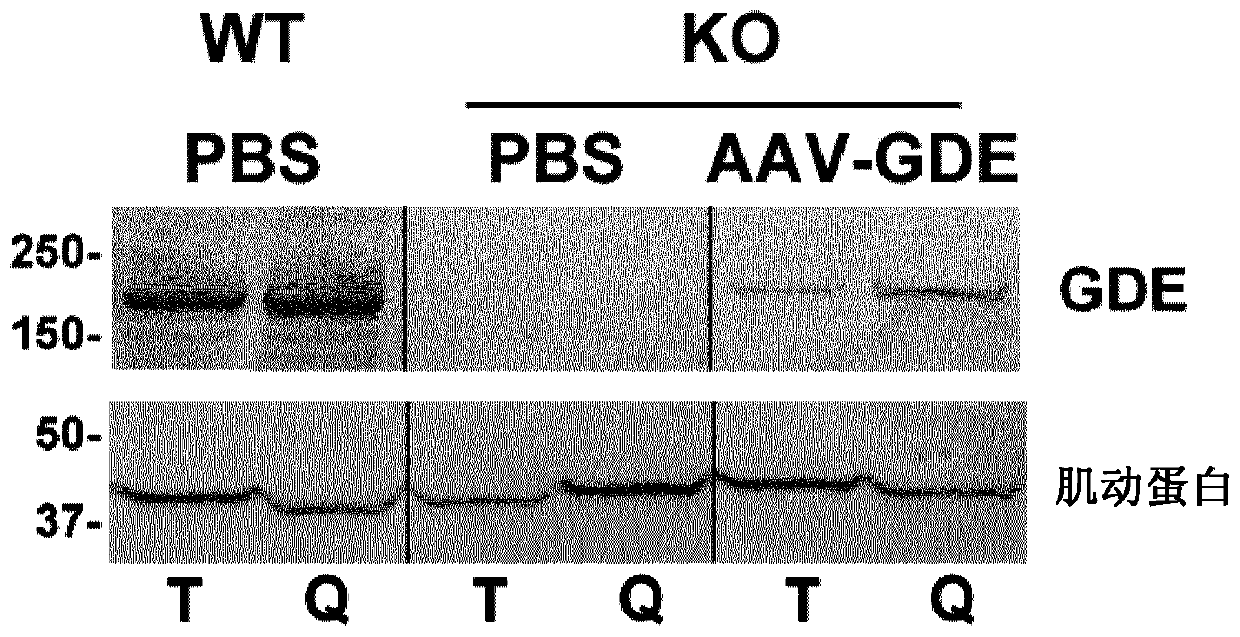
Treatment of glycogen storage disease iii
A nucleic acid sequence and encoding technology, applied in gene therapy, viruses, metabolic diseases, etc., can solve problems such as lack of systematic research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0199] The present invention is described in further detail by referring to the following experimental examples and accompanying drawings. These examples are provided for illustrative purposes only and are not intended to be limiting.
[0200] Materials and methods
[0201] Glycogen content
[0202] Glycogen content was measured indirectly as glucose released after complete digestion with amyloglucosidase. Released glucose was determined using a glucose assay kit by measuring absorbance at 540 nm using an EnSpire alpha plate reader.
[0203] Western blot analysis
[0204] Mouse tissues were prepared as previously described (Amalfitano et al., PNAS 1999). Briefly, 50-100 mg of tissue was weighed and homogenized in DNAse / RNAse-free water, then centrifuged at 10,000 x g for 20 minutes. The supernatant was used in the next step. Protein concentrations were determined using the BCA protein assay. SDS-page electrophoresis was performed on a 4-15% gradient polyacrylamide gel. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com