Methods and compositions for treating glycogen storage diseases
A composition and genome technology, applied in the direction of gene therapy, botanical equipment and methods, biochemical equipment and methods, etc., can solve the incomplete prevention of hyperlipidemia, hyperuricemia, lactic acidemia and liver fat Accumulated, incurable GSD-Ia and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-A
[0116] Example 1 - AAV vectors and rAAV produced from vectors
[0117] AAV vector
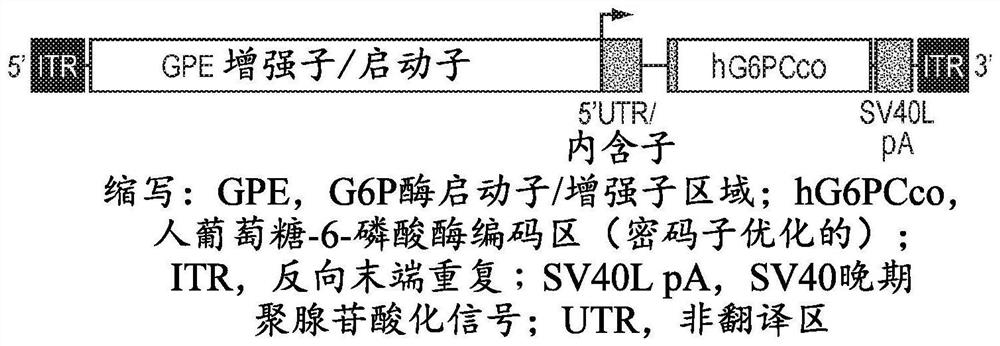
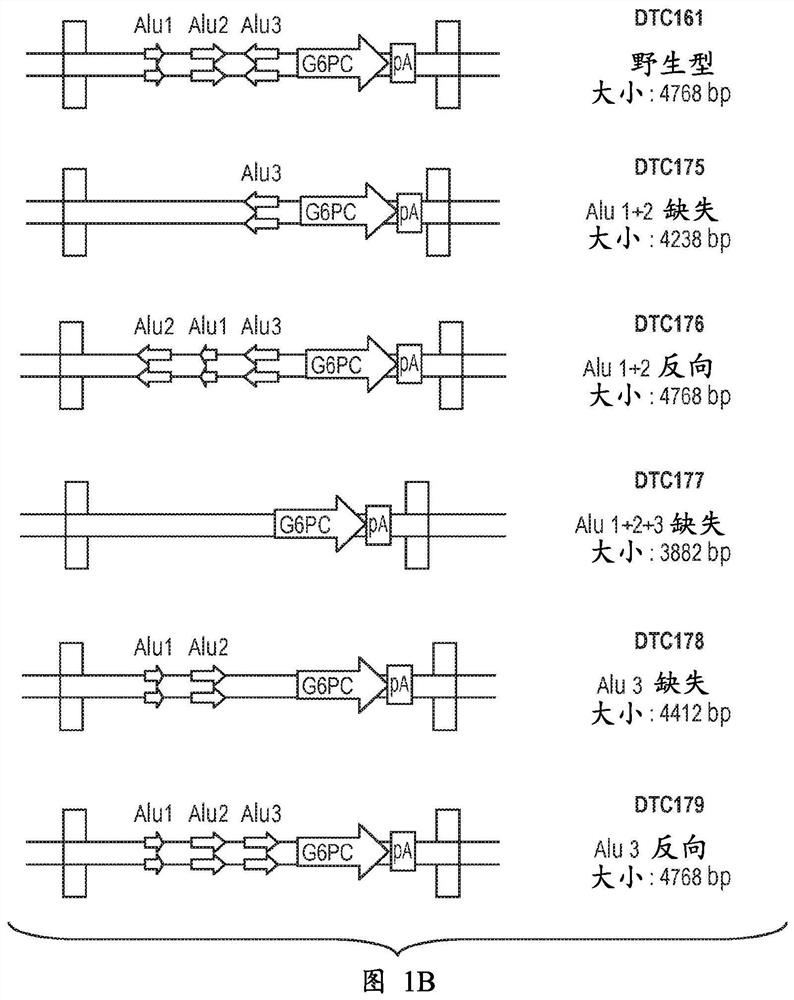
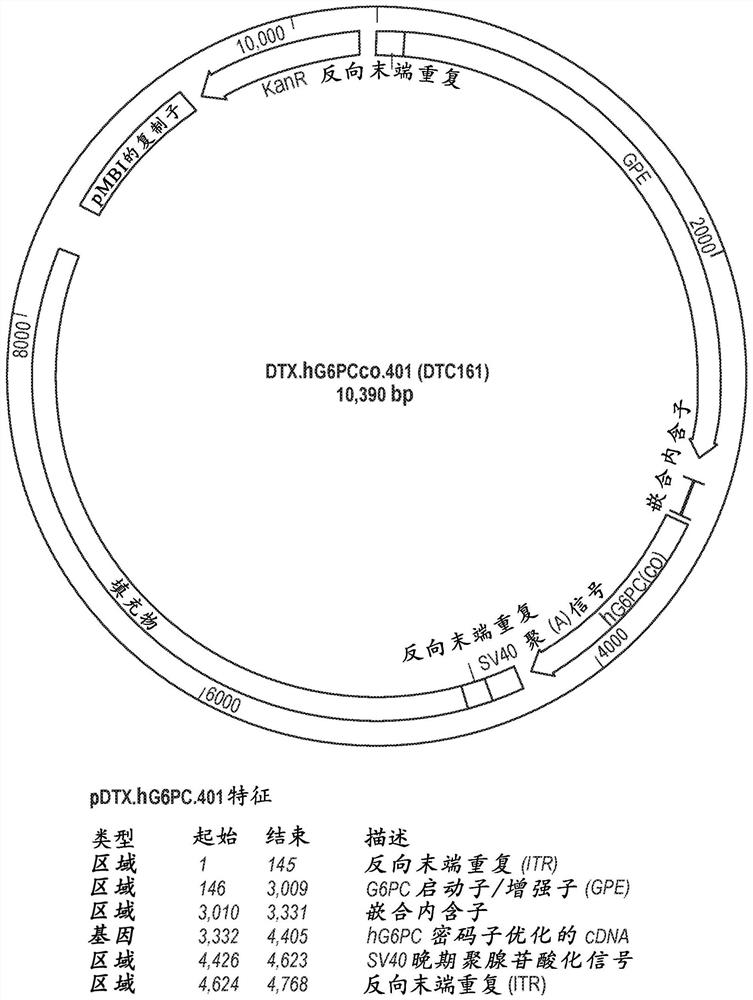
[0118] An AAV vector containing a G6PC expression cassette surrounded by two AAV2 inverted terminal repeats (ITR, SEQ ID NO: 15) was constructed. The primer sequence "1S" listed in Yiu et al., 2010, Molecular Therapy [Molecular Therapy] 18 (6): 1076-84 and its related G6PC promoter / enhancer (GPE) 5' end KpnI restriction endonuclease site definition. By alignment with the SV40 genome and associated SalI restriction endonuclease sites, the G6PC expression cassette is defined at the 3' end by its SV40 late polyadenylation signal. All G6PC expression cassettes contain GPE, introns, codon-optimized human G6PC gene, and SV40 late poly-A tail, as in Figure 1A shown. Different versions of the G6PC expression cassette were produced, each version containing either wild-type GPE or modified GPE, such as Figure 1B shown. The elements of the G6PC expression cassette are described below.
[0119] T...
Embodiment 2
[0128] Example 2-Alu element deletion improves rAAV production
[0129] AAV vectors containing wild-type GPE or modified GPE were used to transfect HEK293 cells with the Rep / Cap plasmids and helper plasmids described above.
[0130] The Alu element was deleted (in DTC175, DTC177 and DTC178 vectors) or the Alu element was reversed in orientation (in DTC176 and DTC179 vectors) in the modified GPE. The DTC175 viral vector contains a G6PC expression cassette containing GPE in which the Alu-1 and Alu-2 sequences have been deleted, and the DTC176 viral vector contains a G6PC expression cassette containing a GPE with all three Alu elements Both exist, but the orientation of the Alu-1 and Alu-2 sequences is reversed, and the DTC177 viral vector contains a G6PC expression cassette comprising GPE in which the Alu-1, Alu-2 and Alu-3 sequences are deleted, The DTC178 viral vector comprises a G6PC expression cassette comprising GPE in which the Alu-3 sequence has been deleted, and the DTC...
Embodiment 3-A
[0134] Example 3 - Alu element deletion improves rAAV packaging
[0135] To assess whether deletion of one or more Alu elements affected rAAV packaging, rAAV produced as described in Example 2 were harvested and extracted from each rAAV (produced from control viral vectors DTC161, DTC175, DTC176, DTC177, DTC178, or DTC179). Total DNA was isolated. about 7.12x10 10 Total amounts of each rAAV GC were subjected to agarose gel electrophoresis followed by SYBRGold staining. The control viral vector used in this experiment was AAV8-LSP-hFIXco3-WPRE-pA (produced at Virovek, custom purified, 150282 cat / lot 061015), which provides the known DNA size on the gel migration, and confirmed that the experimental approach was able to disrupt the integrity of the AAV capsid and release the packaged DNA.
[0136] Such as Figure 7 As shown, the full-length viral DNA is between 3.8kb-5kb. "*" indicates the complete genome of full-length DNA isolated from a control viral vector after capsid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com