New application of recombinant human thymosin alpha collagens
A technology of thymosin and proprotein, applied in the field of recombinant human thymosin alpha proprotein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] The following embodiments will further illustrate the present invention in conjunction with the drawings.
[0019] For the recombinant protein monomer and fusion protein of the present invention, please refer to the applicant's Chinese patent 200810072084.4 (application of a recombinant protein in the preparation of oral medicine for preventing diabetes) and Chinese patent 200710009083.0 (a preparation method of an anti-tumor fusion protein) And its applications).
[0020] Anti-fatigue test of prothymosin alpha monomer and GST-prothymosin alpha fusion protein. Specific steps are as follows:
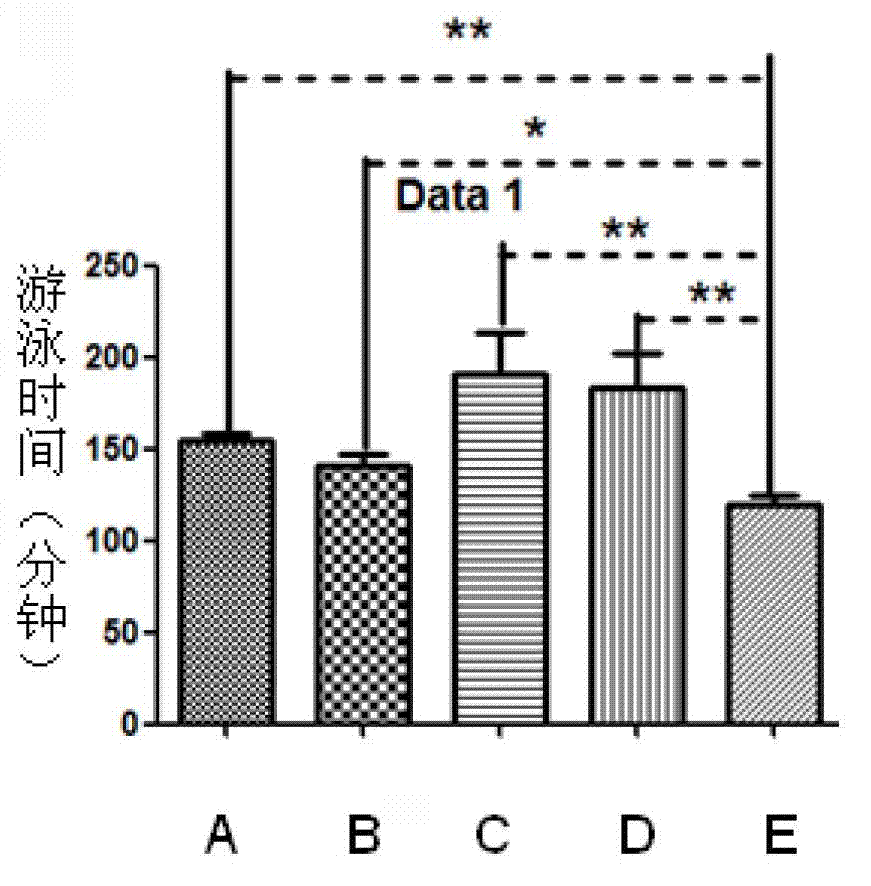
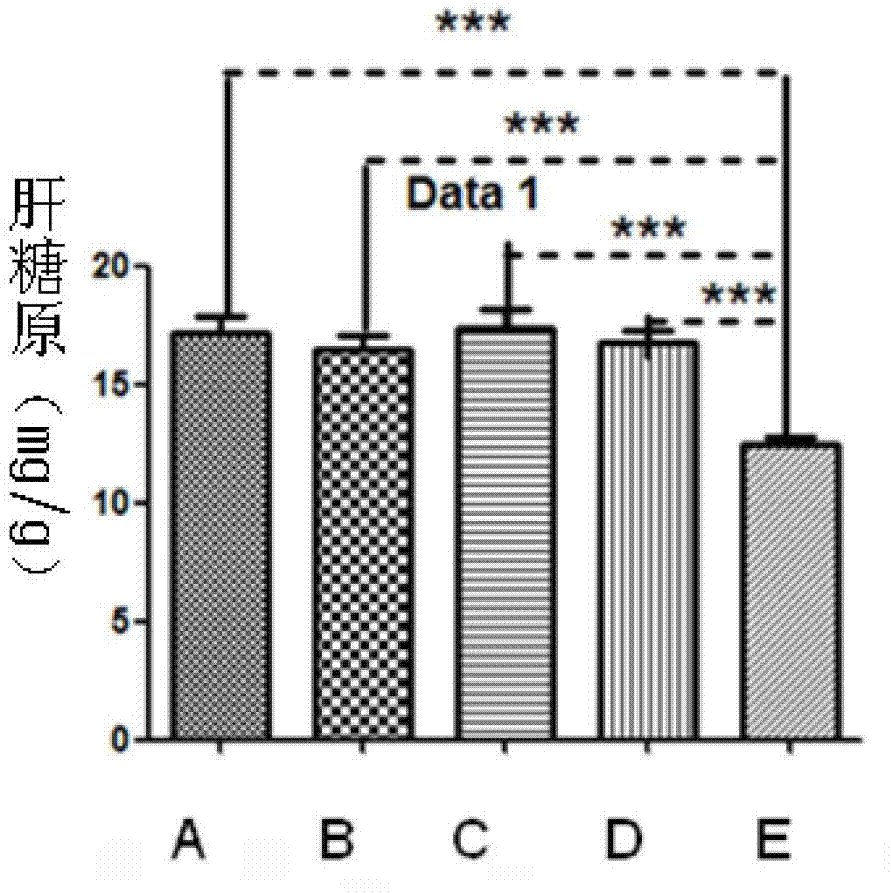
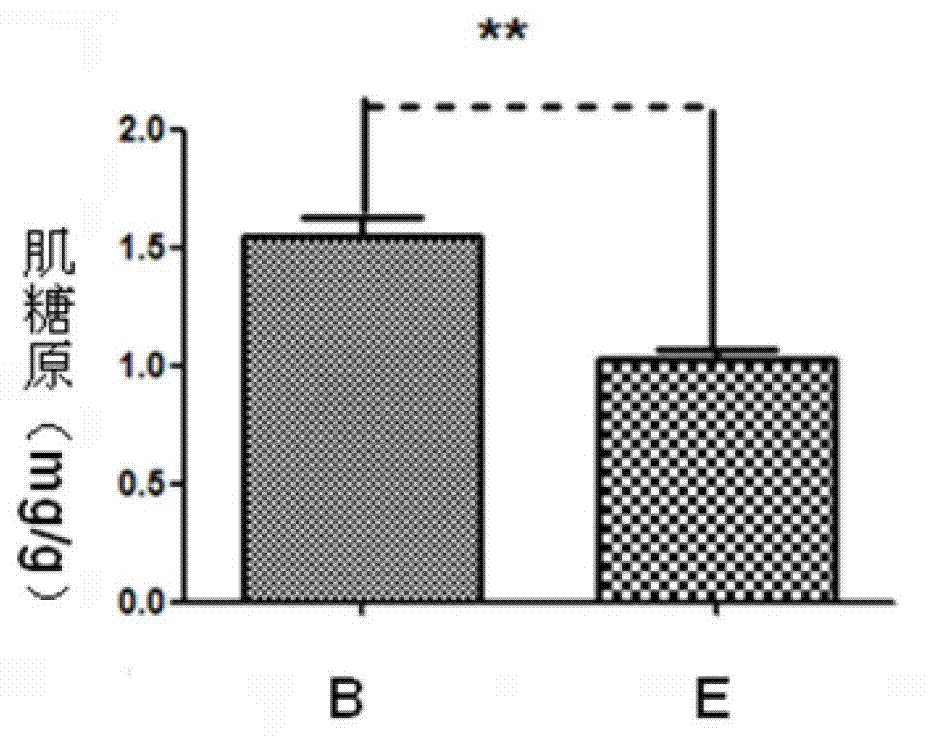
[0021] ① 4 to 5 weeks old Kunming mice were selected and divided into 5 groups, A, B, C, D, E group, E group blank control group, injected the same volume of PBS, A, B, each group injected 1μg / d thymus Pro-thymosin alpha monomer and GST-pro-thymosin alpha fusion protein, groups C and D were injected with 10μg / d of pro-thymosin alpha monomer and GST-pro-thymosin alpha fusion protein, pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com