Adeno-associated virus vectors encoding modified g6pc and uses thereof
A carrier and encoding technology, applied in the direction of viruses/phages, medical preparations containing active ingredients, and the use of vectors to introduce foreign genetic materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
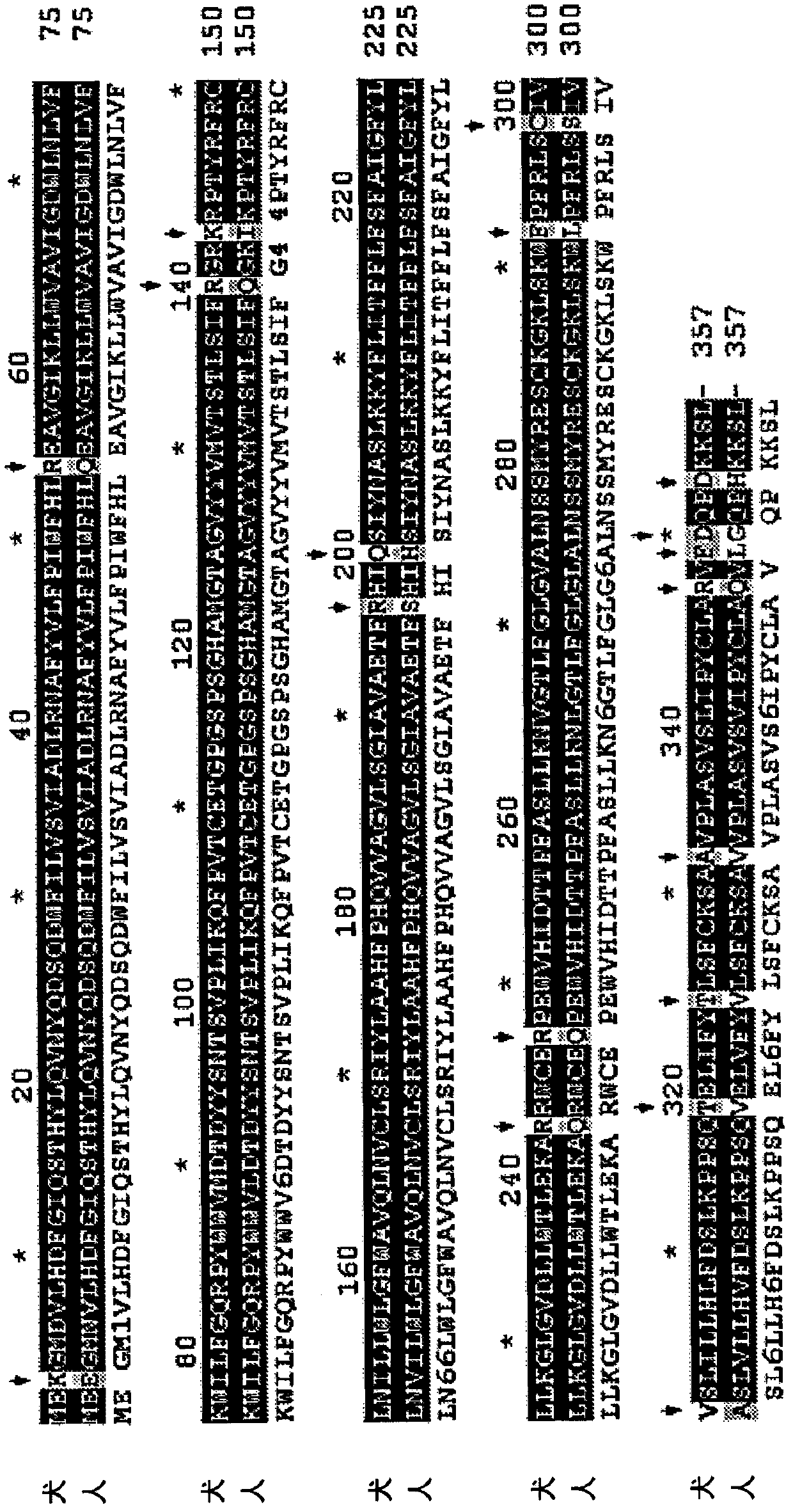
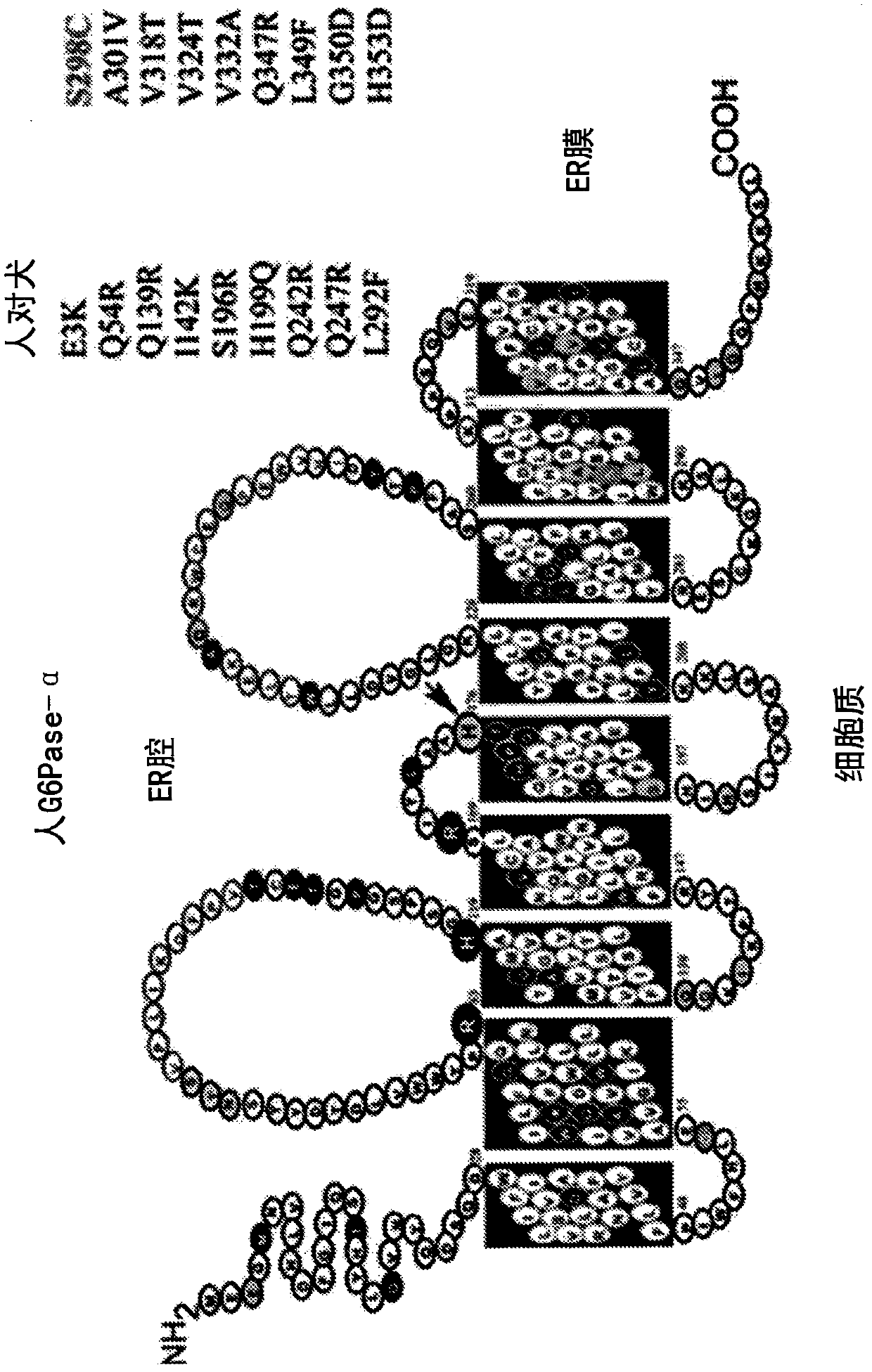
[0164] Example 1: Construction and characterization of human G6PC (G6Pase-α) mutants for AAV-mediated gene therapy
[0165] This example describes the generation of 18 human G6PC mutants and the identification of specific G6Pase-α mutants with increased phosphohydrolase activity.
[0166] Construction of G6PC mutant
[0167] To construct the human G6PC mutant, the pSVL vector containing nucleotides 1 to 1074 of human G6PC cDNA (the entire coding region, where the start codon ATG is at nucleotides 1-3; SEQ ID NO: 11) was used as a template . For PCR-directed mutagenesis, two external PCR primers are used to amplify the template. The external primers match nucleotides 1 to 20 (sense) and 1055 to 1074 (antisense), located in a 20-nucleotide long sense and The outer side of the antisense mutant primer (flanked), where the codon to be mutated is in the middle of the mutant primer (see Figure 4 And Table 1) below. The template of the hG6PC-S298C / A301V double mutant is the pSVL-hG6PC-S2...
Embodiment 2
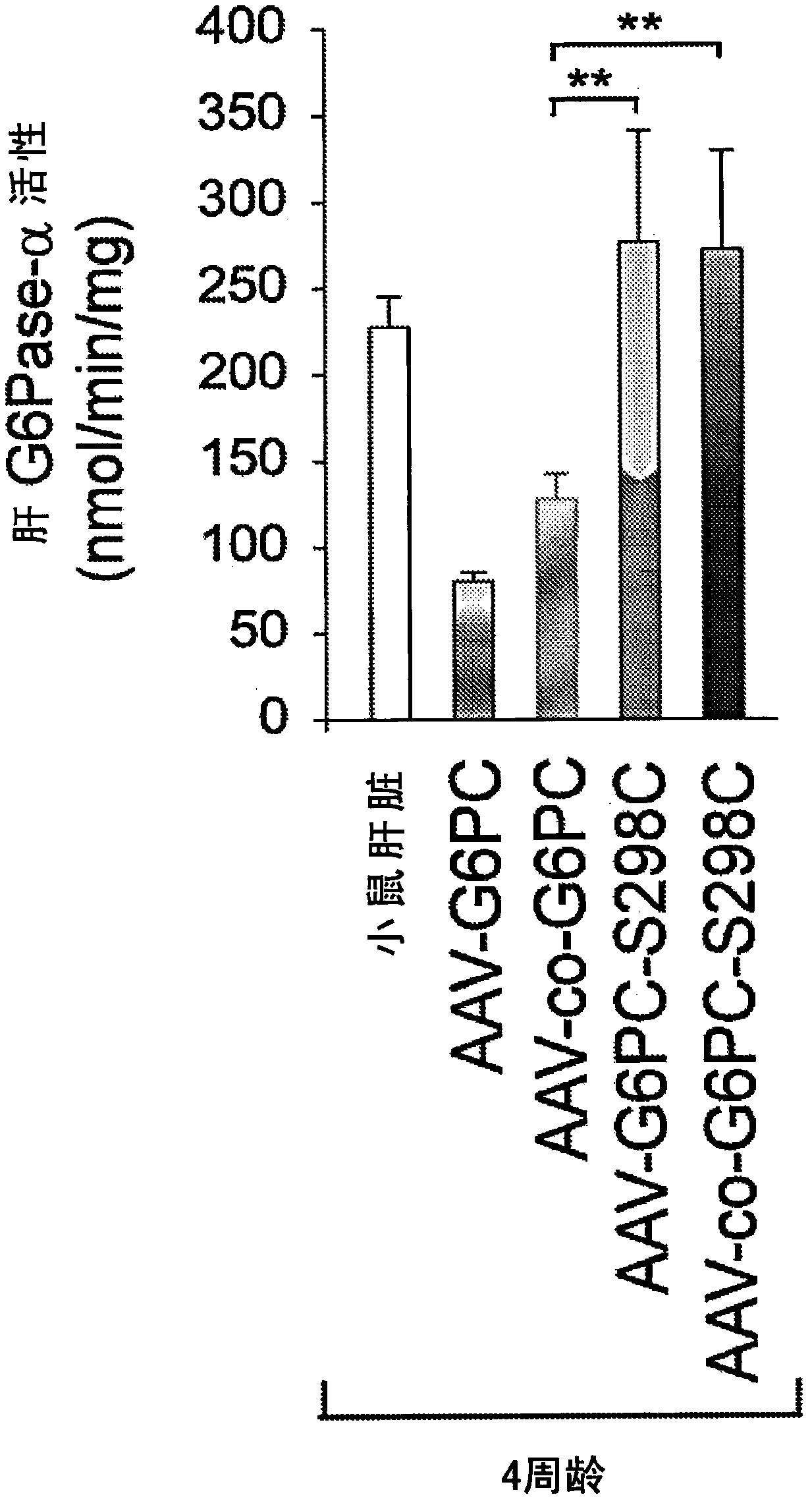
[0189] Example 2: Evaluation of the minimum carrier dose required to correct liver G6Pase-α deficiency
[0190] This example describes a study to determine the minimum dose required to restore G6Pase-α activity to a level that prevents the development of HCA / HCC and maintains glucose homeostasis.
[0191] GSD-Ia is characterized by impaired glucose homeostasis and long-term complications hepatocellular adenoma (HCA) (Chou et al., Nat Rev Endocrinol 6:676-688, 2010). The inventors have previously demonstrated that G6pc- / - mice treated with rAAV8-G6PC express ≥3% of normal liver G6Pase-α activity (which is equivalent to ≥5 units of G6Pase-α activity; 1 nmol / min / mg It is defined as a unit of G6Pase-α activity), which maintains glucose homeostasis to P70-P90 weeks of age and does not develop HCA (Lee et al., Hepatology 56: 1719-1729, 2012; PCT Publication No. WO 2015 / 081101, It is incorporated herein by reference).
[0192] This study was performed using purified rAAV8 vector (supplied...
Embodiment 3
[0200] Example 3: Treatment of human GSD-Ia using AAV-based gene therapy
[0201] This example describes an exemplary method of clinically using AAV vectors encoding modified G6PC to treat GSD-Ia.
[0202] Select patients diagnosed with GSD-Ia for treatment. Usually the patient is at least 18 years old and may or may not be pre-exposed to immunomodulation. The patient is administered a therapeutically effective amount of a recombinant AAV expressing modified G6PC, such as rAAV comprising SEQ ID NO: 4 or SEQ ID NO: 5, as described herein. Recombinant AAV can be administered intravenously. The appropriate therapeutic dose can be selected by the physician. In some cases, the therapeutically effective dose is 1×10 10 To 1×10 14 Virus particles (vp) / kg, such as about 1×10 11 Or 1×10 12 vp / kg. In most cases, a single dose is administered to the patient. In the absence of immune regulation, patients may only tolerate a single infusion of rAAV. If the subject is previously exposed to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com