Sustained-release glucose preparation for treating glycogen storage diseases
A sustained-release preparation and glucose technology, which are applied in the field of glucose sustained-release preparations for treating glycogen storage disease and their preparation, can solve the problems of volume expansion, many taking times, deviation of therapeutic effects, etc., and achieve the effect of good sustained-release performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Instruments and equipment:
[0060] Digital Overhead Mechanical Stirrer (RW20)
[0061] Fluidized bed granulation dryer (FLZB1.5, Chuangzhi Electromechanical)
[0062] INNOJET fluidized bed (VENTILVSL, INNOJET, Germany)
[0063] Extrusion spheronizer (S-250, Chongqing Yingge Granulation Coating Technology Co., Ltd.)
[0064] Low temperature rotary extruder (LTAE-200, Shenzhen Xinyite Technology)
[0065] Wet mixing granulator (HLSHZ-6, Beijing Institute of Aeronautical Manufacturing Engineering)
[0066] Analytical Balances
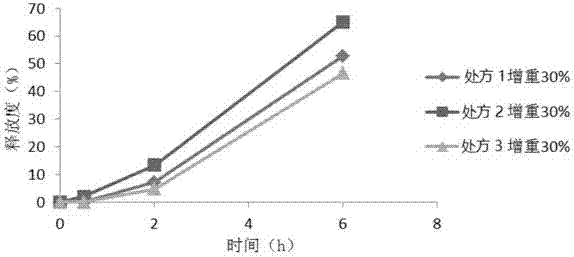
[0067] Prescription one:
[0068] Raw material weight ratio:
[0069] Glucose pellet core: glucose monohydrate 360; microcrystalline cellulose-101 40;
[0070] Film Coating: Ethylcellulose-20P 120; Hydroxypropylcellulose-EF 15;
[0071] Triethyl citrate 12; 95% ethanol 1800;
[0072] Purified water 450.
[0073] The preparation method is as follows:
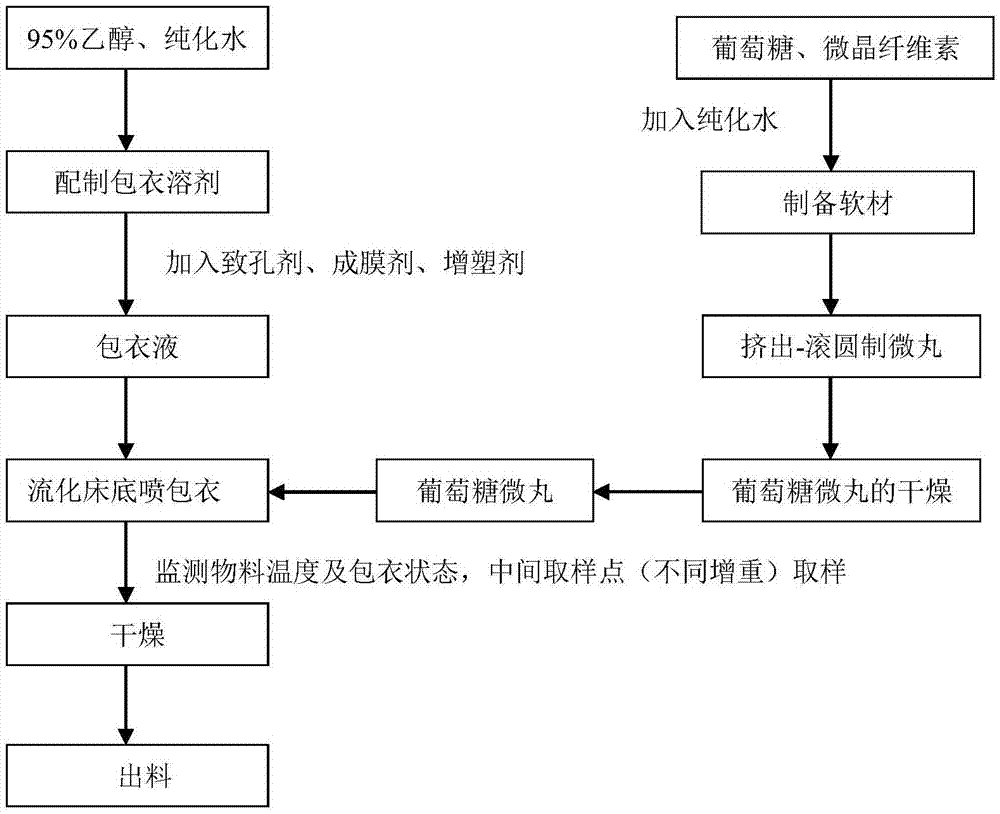
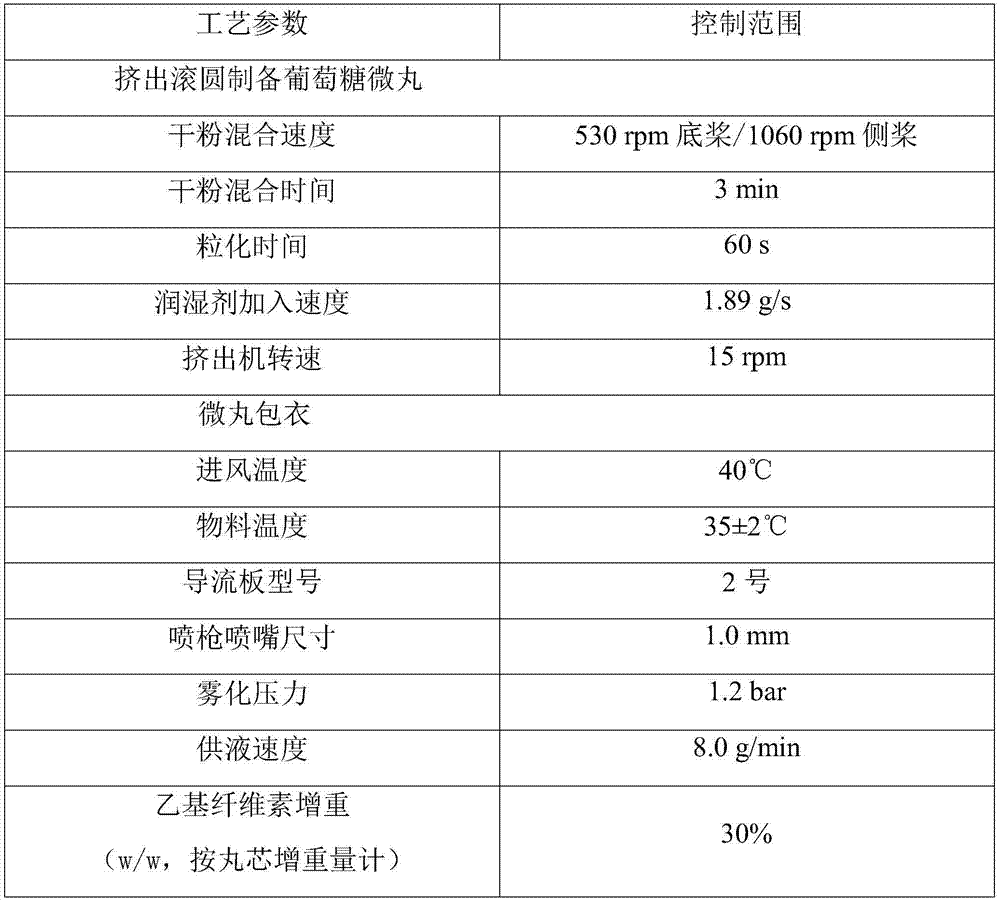
[0074] 1) Preparation of glucose pellets:
[0075] ① Preparation of soft material: Wei...
Embodiment 2
[0087] Instrument and equipment: with embodiment 1.
[0088] Prescription two:
[0089] Raw material weight ratio:
[0090] Glucose pellet core: glucose monohydrate 360; microcrystalline cellulose-101 36;
[0091] Film Coating: Ethylcellulose-20P 120; Hydroxypropylcellulose-EF 30;
[0092] Triethyl citrate 12; 95% ethanol 2300;
[0093] Purified water 575.
[0094] The preparation method is as follows:
[0095] 1) Preparation of glucose pellets:
[0096] ① Preparation of soft material: Weigh the prescribed amount of glucose monohydrate and microcrystalline cellulose-101 (as diluent) into a wet mixing granulator, mix for 10 minutes, select purified water as the wetting agent, and It is added in the form of atomization, and the soft material can be obtained after granulation;
[0097] ②Extrusion--spheronizing pellets: Take appropriate amount of soft materials and put them into the extruder to prepare extruded materials, put the extruded materials into the spheronizer, adj...
Embodiment 3
[0107] Instrument and equipment: with embodiment 1.
[0108] Prescription three:
[0109] Raw material weight ratio:
[0110] Glucose pellet core: glucose monohydrate 360; microcrystalline cellulose-101 40;
[0111] Film Coating: Ethylcellulose-45P 80; Hydroxypropylcellulose-EF 10;
[0112] Triethyl citrate 8; 95% ethanol 2055;
[0113] Purified water 515.
[0114] The preparation method is as follows:
[0115] 1) Preparation of glucose pellets:
[0116] ① Preparation of soft material: Weigh the prescribed amount of glucose monohydrate and microcrystalline cellulose-101 (as diluent) into a wet mixing granulator, mix for 5 minutes, use purified water as the wetting agent, and It is added in the form of atomization, and the soft material can be obtained after granulation;
[0117] ②Extrusion--spheronizing pellets: Take appropriate amount of soft materials and put them into the extruder to prepare extruded materials, put the extruded materials into the spheronizer, adjust ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com