Medicament combination preparation for treating bacterial infection
A bacterial and drug technology, applied in the field of drugs for the treatment of bacterial infections, can solve the general trend of society that is not conducive to reducing medical costs, reduce the dosage of antibiotics, and increase the cost of treatment, and achieve beneficial bacterial infections, maintain curative effects, and significantly improve society. Benefit and economic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
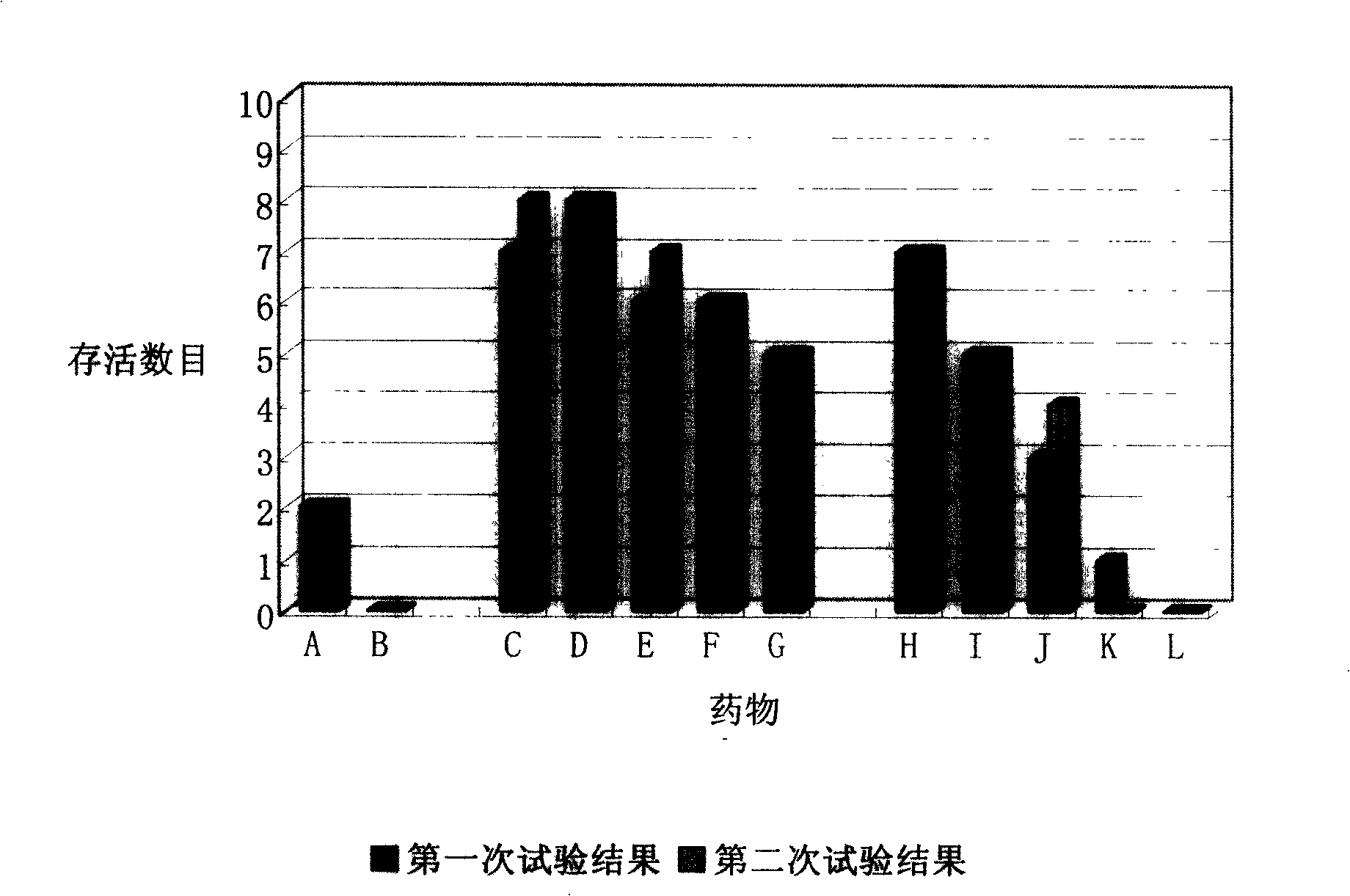
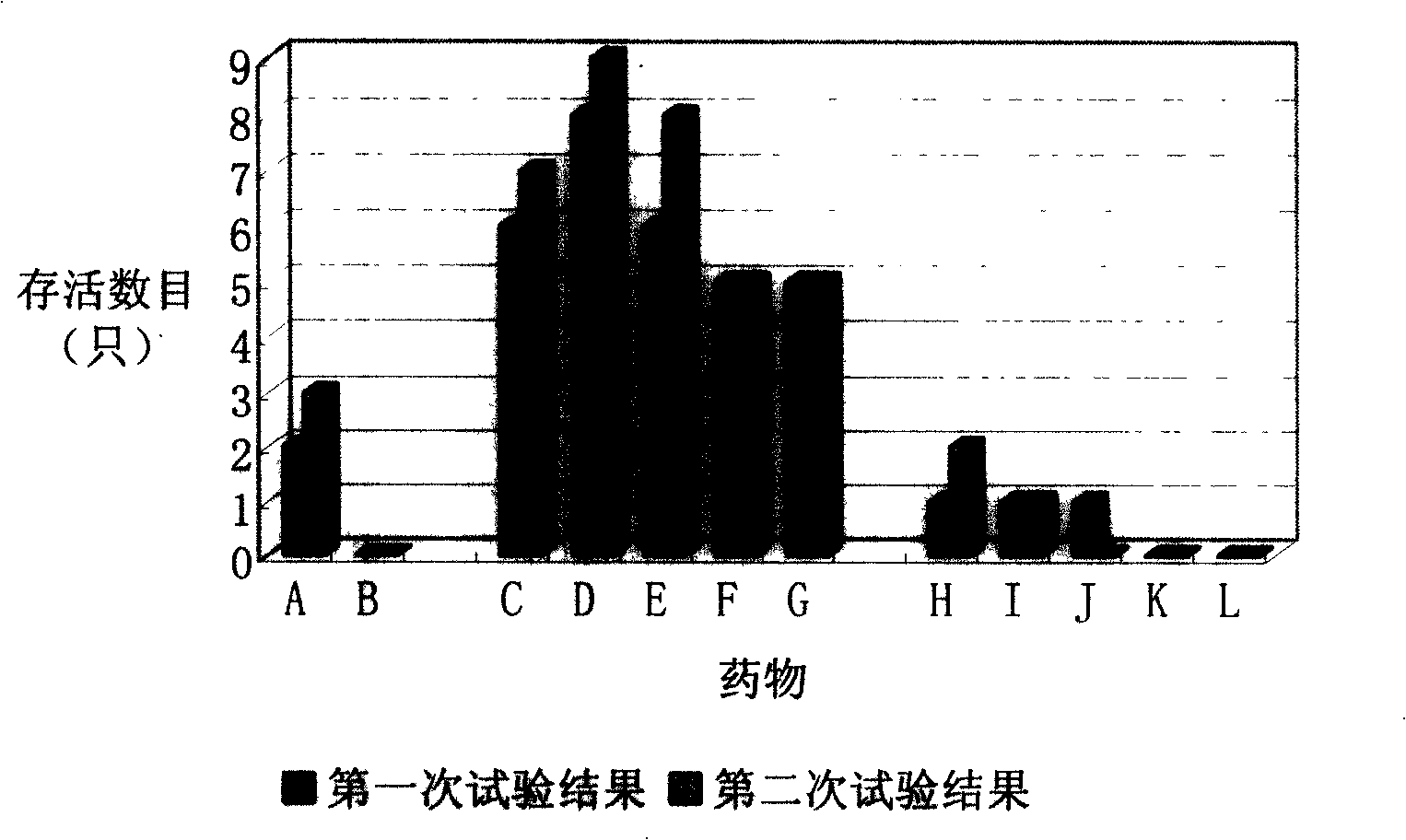
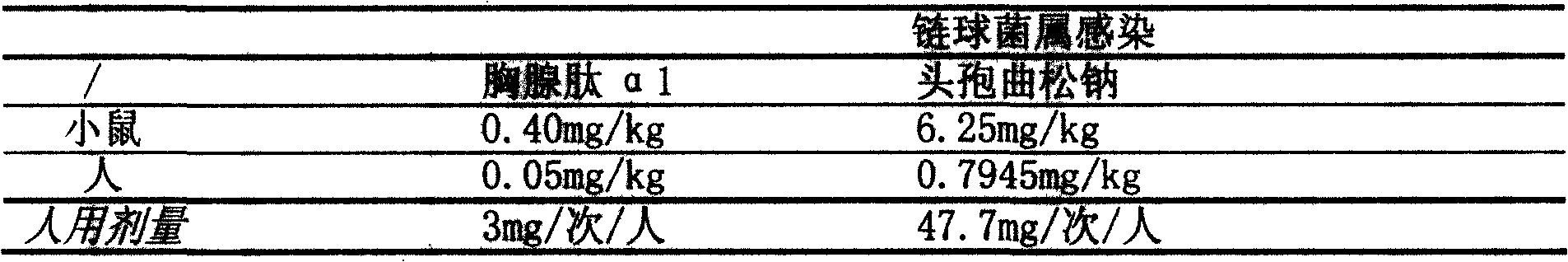
Image
Examples
Embodiment 1
[0033] Embodiment 1 Thymosin α1 and ceftriaxone sodium medicine combined preparation
[0034] Each vial contains 1 mg of thymosin α1 sterile freeze-dried powder, 7 in total; each vial contains 30 mg of sterile ceftriaxone sodium dry powder, 2 in total, put them in the same package, and use them together according to the instructions. The thymosin α1 and ceftriaxone sodium both meet the requirements of the 2005 edition of the Chinese Pharmacopoeia.
Embodiment 2
[0035] The drug combination preparation of embodiment 2 thymosin alpha 1 and ceftriaxone sodium
[0036] Each vial contains 3 mg of thymosin α1 sterile freeze-dried powder, a total of 7; each vial contains 47.5 mg of sterile ceftriaxone sodium dry powder, a total of two, put in the same package, and use them together according to the instructions. The thymosin α1 and ceftriaxone sodium both meet the requirements of the 2005 edition of the Chinese Pharmacopoeia.
Embodiment 3
[0037] The drug combination preparation of embodiment 3 thymosin alpha 1 and ceftriaxone sodium
[0038] The independently packaged preparation contains 10 vials, and each bottle contains 3 mg of thymosin α1 sterile freeze-dried powder. The built-in instructions and the label on the bottle indicate that the preparation is used in combination with 60 mg of ceftriaxone sodium. The independently packaged preparation has a built-in vial containing 60 mg of dry powder of sterile ceftriaxone sodium, and the built-in instructions and the label on the bottle indicate that the preparation is used in combination with 3 mg of thymosin α1. The thymosin α1 and ceftriaxone sodium both meet the requirements of the 2005 edition of the Chinese Pharmacopoeia.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com