Thymosin alpha peptide for preventing, reducing the severity of, and treating infection
A Thymosin, Severity Technology, Applied in the Field of Infections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
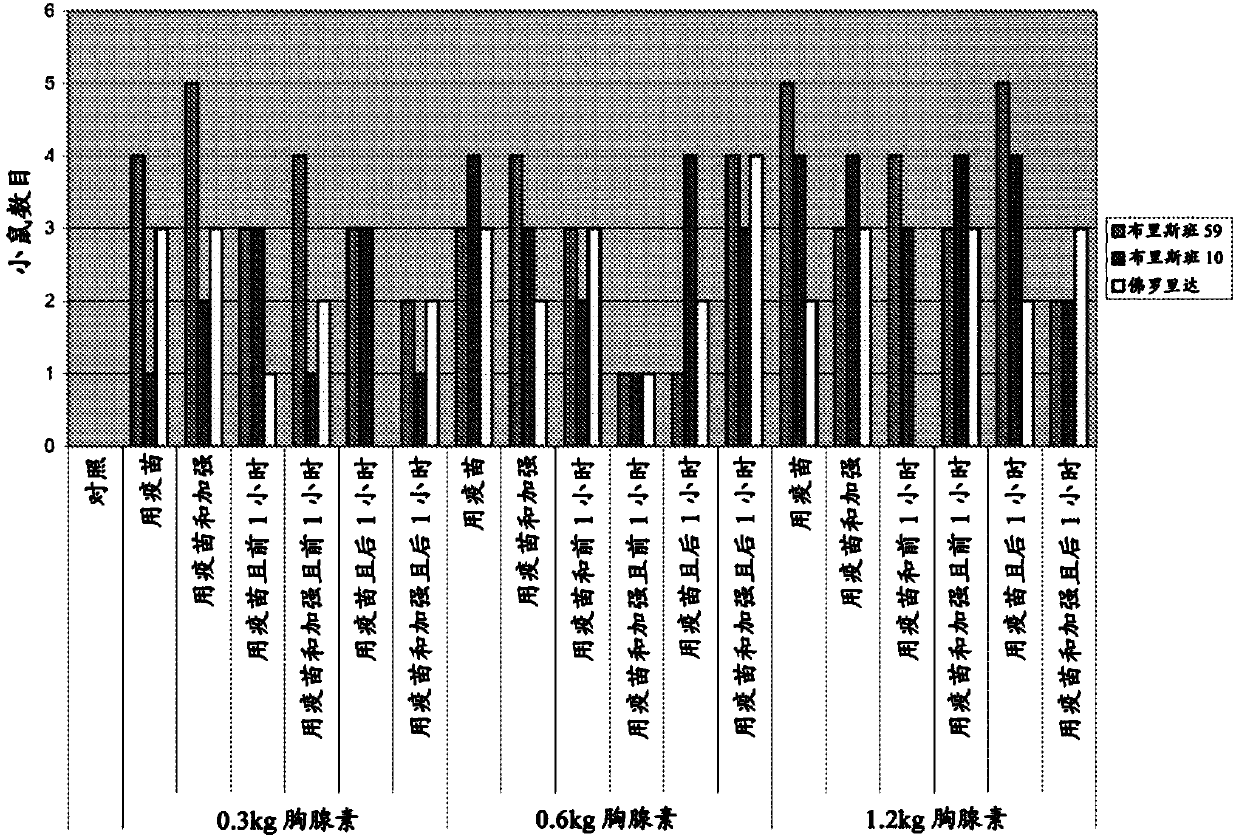
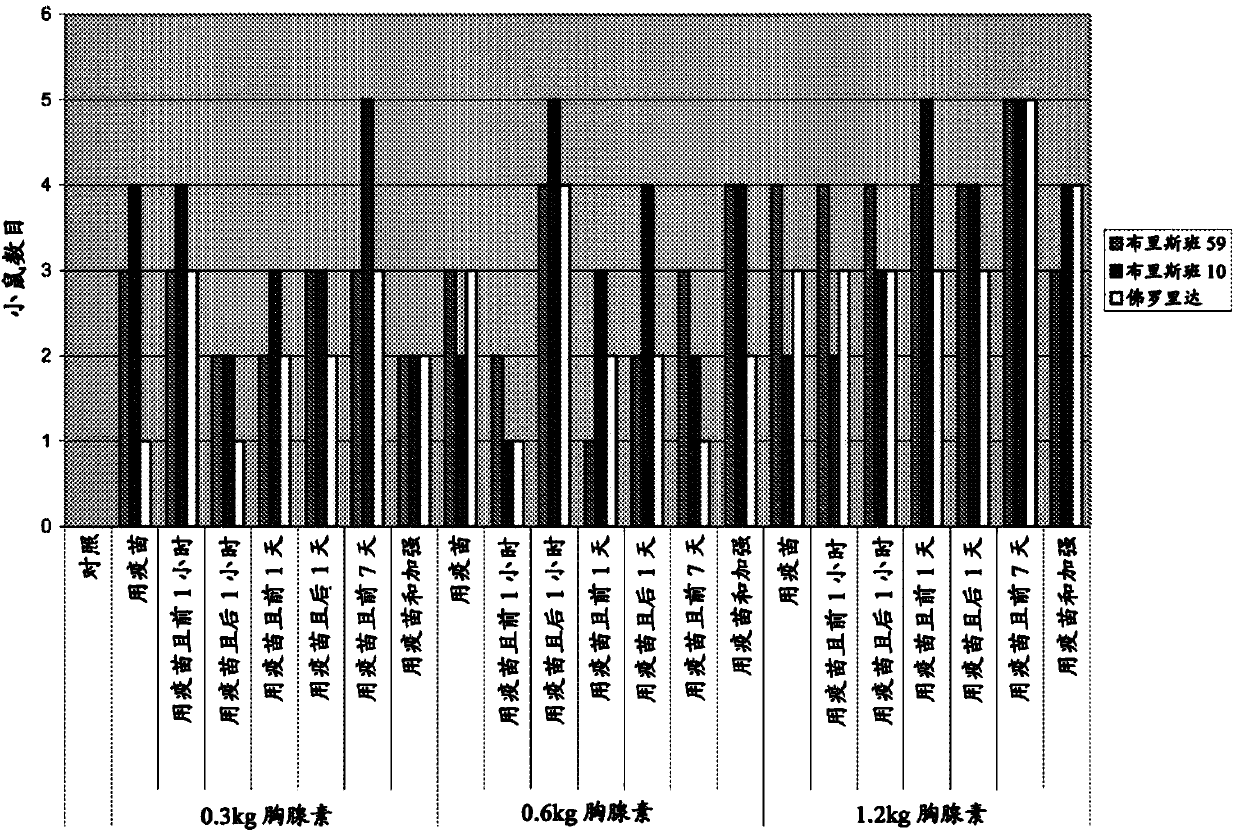
[0078] Example 1: Enhancement of H1N1 Vaccination in Mice
[0079] summary
[0080] A study was conducted to determine the role of TA1 (thymalfasin) in seasonal influenza vaccines Potential to enhance anti-influenza antibody formation in CD-1 mice following different vaccination schedules in 2008-2009. Mice received control articles or vaccines on study days (SD) 1 and 10 or SD 8 and 17. Mice also received different doses of TA1 at different times relative to vaccine administration. Both the control article and the vaccine were administered via intramuscular injection to both the right and left hind limbs; TA1 was administered by the intraperitoneal route. All mice were given a fixed dose of control / vaccine regardless of body weight. Mice were observed twice daily for mortality, moribundity, general health, and signs of toxicity; body weights were recorded prior to dosing. Blood samples were collected at SD 20 or 27 (10 days after the final vaccine administration) and ...
Embodiment 2
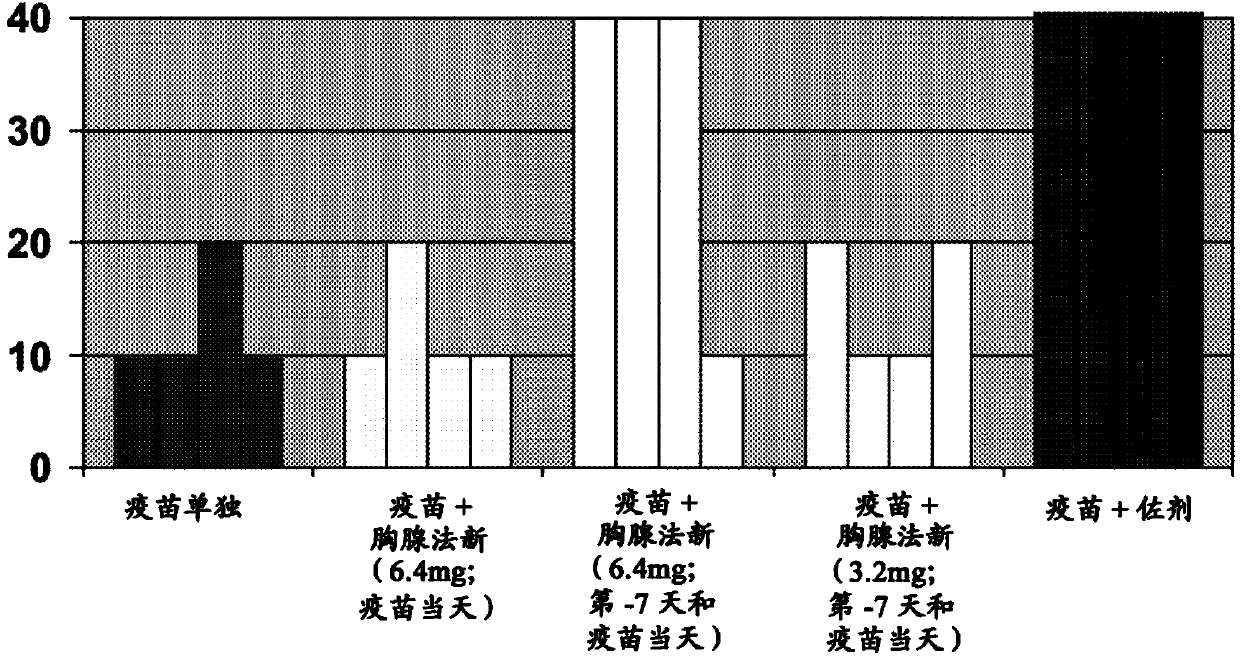
[0107] Example 2: Enhancement of H1N1 Vaccination in Ferrets
[0108] Thymosin has been shown to exert immune regulation in several microbial and tumor settings through a variety of mechanisms, which include enhancing antibody responses. To control the current influenza pandemic caused by the novel A / H1N1 virus of porcine origin, mass active vaccination will be performed in most countries and vaccines with or without adjuvant will be used. At least some of these vaccines will require a booster dose 1 month later to induce detectable virus-neutralizing antibody production in most vaccinated individuals. Furthermore, the availability of these vaccines to the entire target population is doubtful. Therefore, it is important to assess whether an appropriate dose of thymosin administered separately but concurrently with influenza vaccine can boost the antibody response against the virus.
[0109] Experimental Study
[0110] Influenza-free ferrets are very responsive to influenz...
Embodiment 3
[0120] Example 3: Boosters of H1N1 Vaccination in Hemodialysis Patients
[0121] Investigating the effect of thymosin TA1 enhancement on the H1N1 influenza monovalent vaccine Focetria adjuvanted with MF59 TM ability of the immune response. Studies were conducted in hemodialysis patients. Patients with end-stage renal disease requiring hemodialysis or other conditions that compromise the immune system, as well as the elderly, often do not develop enough antibodies to fight off infectious diseases such as the H1N1 flu. Additionally, many patients who achieve protective titers are initially unable to maintain these for longer periods of time, making them susceptible to infection and requiring revaccination or booster injections.
[0122] A randomized three-arm study in approximately 120 patients with end-stage renal disease on long-term dialysis. One subgroup of patients received the H1N1 vaccine alone, while the other two groups received two lower doses of thymofasin (TA1) in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com