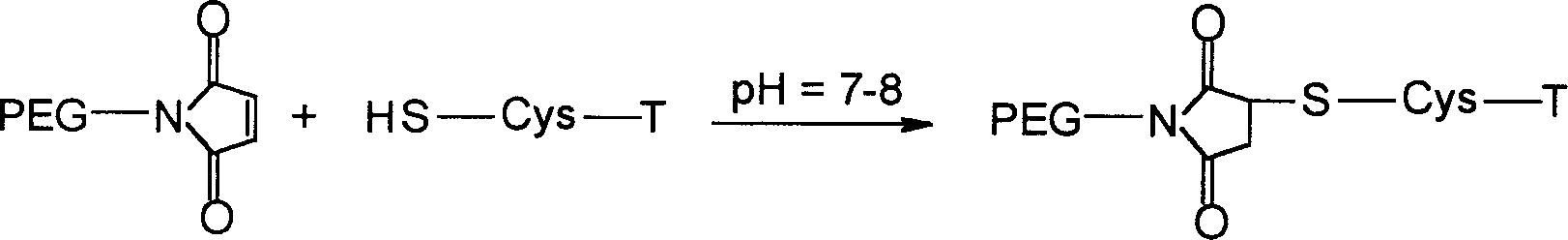Polyethylene glycol derivatives of thymosin alphal
A technology of derivatives and compounds, applied in the field of pegylated derivatives of thymosin α1, which can solve the problems of long cycle, high price, and large dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Example 1 Cys(mPEG 5000 Preparation of -MAL)-Tα1
[0091] 1.1 mPEG 5000 -Synthesis of MAL
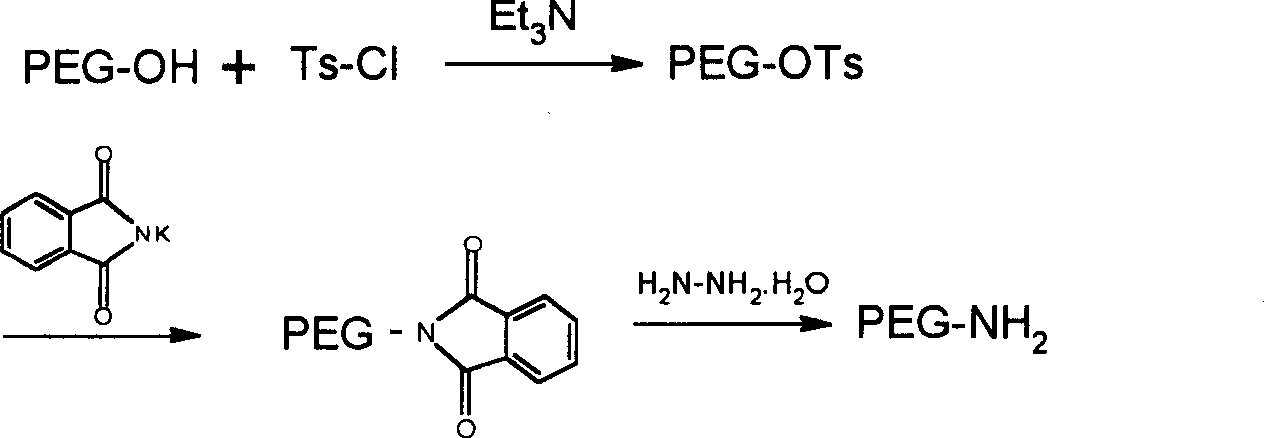
[0092] Weigh mPEG 5000 -OH 20g (4mmol) was placed in a 250ml reaction bottle, and 100ml CH was added 2 Cl 2 , after the solid dissolved, add 3.0ml Et 3 N (20mmmol) and 3.8g Ts-Cl (20mmol), stirred at room temperature. After TLC monitors that the reaction is complete, the solvent is removed by rotary evaporation, and 100 ml of anhydrous ether is added to precipitate a solid to obtain 15.2 g of mPEG 5000 -OTs, yield 70%.
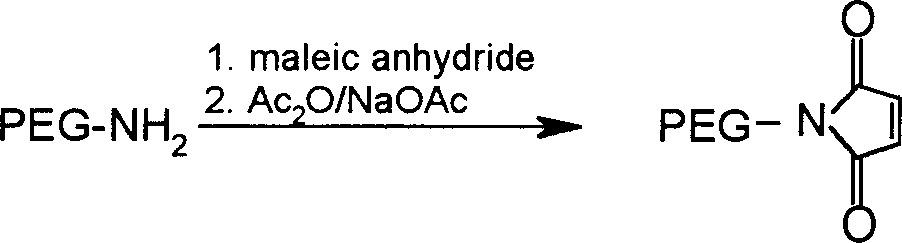
[0093] 15g mPEG 5000 -OTs (3mmol) was dissolved in 30ml DMF, 1.68g (18mmol) phthalimide potassium salt was added, and reacted at 120°C for 4 hours. The solvent was distilled off under reduced pressure, the residue was dissolved in 50 ml of absolute ethanol, 2.0 ml of hydrazine hydrate was added, and the mixture was refluxed for 4 hours. The solvent was removed by rotary evaporation and the residue was dissolved in 30 ml CH 2 Cl 2 , filtered off the in...
Embodiment 2
[0100] Example 2 Tα 1 (17-24)-Cys(mPEG 5000 -MAL)-NH 2 preparation of
[0101] 2.1 Tα 1 (17-24)-Cys-NH 2 Synthesis
[0102] With 0.1mmol Rink amide resin as carrier, Fmoc-AA-OH (0.2mmol) as raw material, HBTU (0.2mmol)-NMM (0.3mmol) as condensation agent, according to Tα 1 The amino acid sequence of (17-24) was synthesized into a fully protected peptide resin. Use EDT-m-cresol-TFA as the lysate, react at 0°C for 90 minutes, filter off the resin, remove the TFA by rotary evaporation of the filtrate, add anhydrous ether to precipitate a white solid, collect the solid, dissolve in water, freeze-dry to obtain a white Dry powder 20mg. RP-HPLC purification, FAB-MS analysis, M+1 peak: 1091 (theoretical value: 1090).
[0103] 2.2 Tα 1 (17-24)-Cys(mPEG 5000 -MAL)-NH 2 Synthesis
[0104] Tα purified by RP-HPLC 1 (17-24)-Cys-NH 2 Dissolve in water, adjust pH to 7-8 with sodium bicarbonate, add 3 times the equivalent of mPEG 5000 -MAL, reacted at room temperature for 2 hour...
Embodiment 3
[0106] Example 3 Tα 1 Induction of IFN-γ produced by mouse splenocytes and its analogues
[0107] The mice were sacrificed by decapitation and bloodletting, and the spleen was quickly removed under aseptic conditions and placed in RPMI1640 solution. Put the spleen on a 100-mesh stainless steel net or nylon net and grind it with a needle core or a triangular glass rod to make a spleen cell suspension. After the splenocytes were centrifuged and washed once with culture medium, Tris-NH was added 4 Cl buffer (0.16M NH 4 Cl and 0.17M Tris were mixed at a ratio of 9:1, pH7.2) to act for 3-5 minutes to dissolve RBC, and then centrifuged and washed twice with culture medium. With trypan blue staining, the number of viable cells should be above 95%. Adjust the cell concentration to 1.25×10 with 10% calf serum 1640 7 A / mL spare. Add splenocytes to a sterile 48-well culture plate, 800 μL per well; then add 100 μL of the samples to be tested at different concentrations shown in Tabl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com