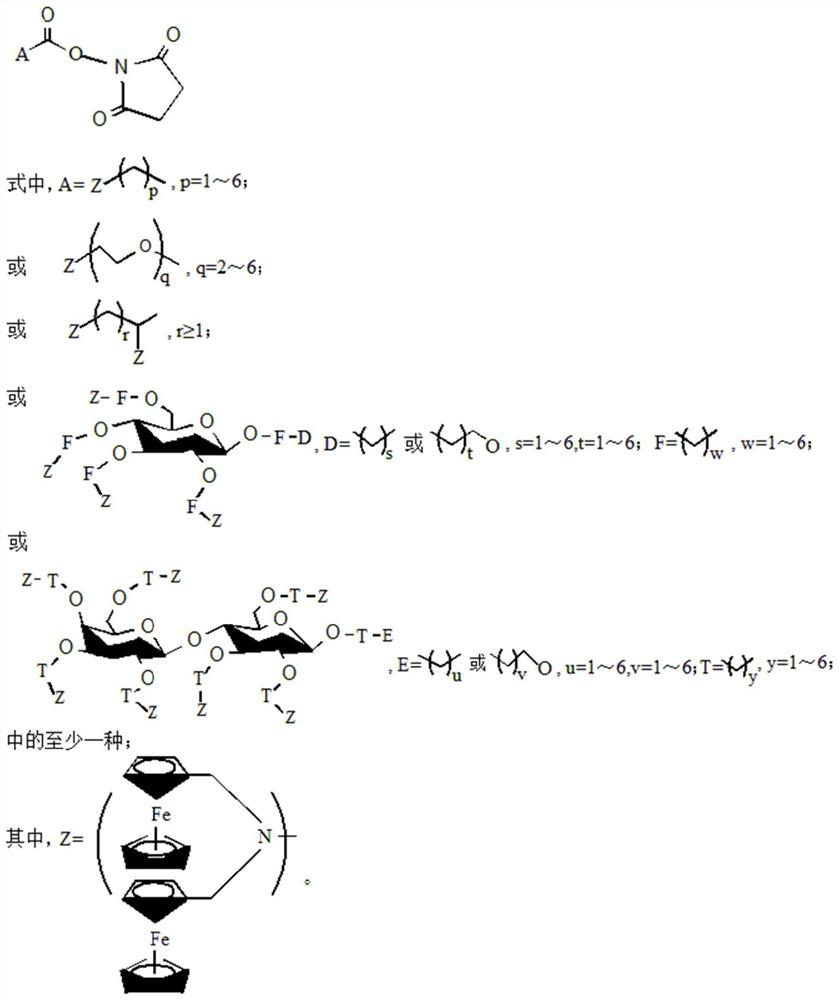
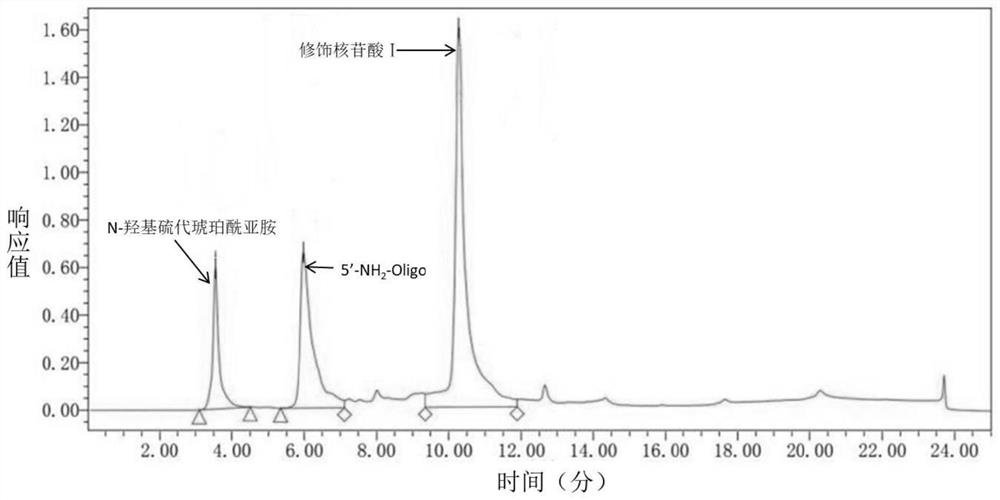
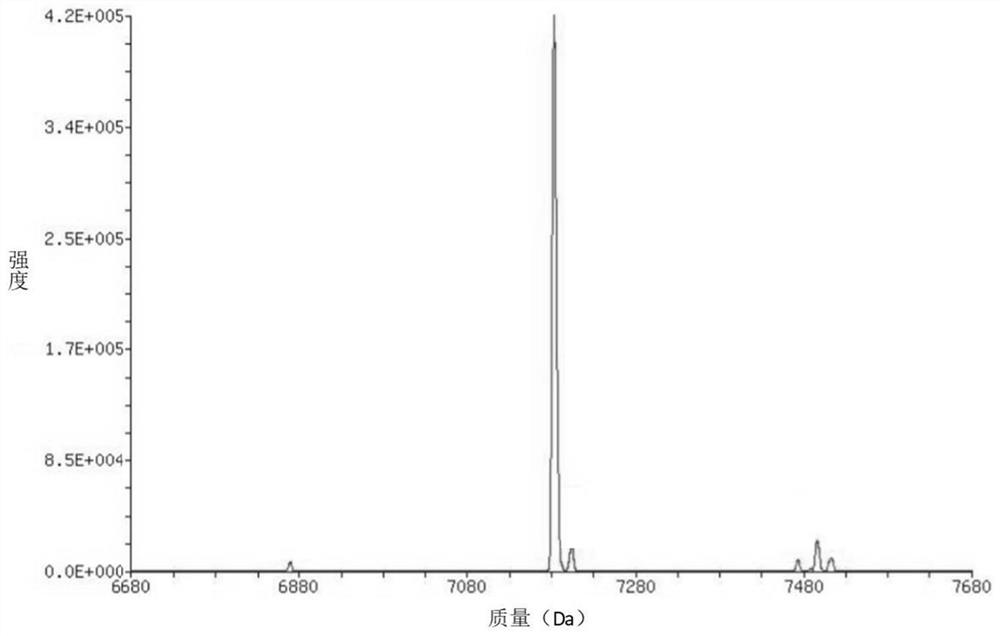
Ferrocene derivative as well as synthesis method and application thereof
A technology of ferrocene derivatives and synthesis methods, applied in the field of organic chemistry synthesis, can solve problems such as detection signal and sensitivity limitation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] This embodiment has prepared a kind of divalent ferrocene derivative, and specific process is:
[0106] (1) Dissolve 0.5g (4.27mmol) of 6-amino-1-hexanol in 25mL of anhydrous tetrahydrofuran, add 2.1g (9.81mmol, 2.31eq) of ferroceneformaldehyde at room temperature and stir, and then take triacetoxy Sodium borohydride 2.3g (10.90mmol, 2.5eq) was added to the system in batches and reacted overnight. After the reaction was detected by thin-layer chromatography (TLC), it was quenched with 30 mL of saturated sodium carbonate solution, extracted with 50 mL of ethyl acetate, then washed with 20 mL of saturated brine and high-purity water successively, and the organic phase was dried over anhydrous sodium sulfate. Concentration, sample mixing and column chromatography gave 1.8 g of 6-N,N-bisferrocenylmethylamino-1-hexanol (dark brown solid), with a yield of 85%.
[0107] The parameters of the structure of 6-N,N-bisferrocenemethylamino-1-hexanol verified by proton nuclear magne...
Embodiment 2
[0112] This embodiment has prepared a kind of tetravalent ferrocene derivative, and specific process is:
[0113] (1) Dissolve 0.5g (3.42mmol) of L-lysine in 25mL of anhydrous tetrahydrofuran, add 3.37g (15.73mmol, 4.6eq) of ferroceneformaldehyde at room temperature and stir, and then take triacetoxy borohydrogenation Sodium 3.62g (17.10mmol, 5eq) was added to the system in batches and reacted overnight. After the reaction was detected by thin-layer chromatography (TLC), it was quenched with 30 mL of saturated sodium carbonate solution, extracted with 50 mL of ethyl acetate, then washed with 20 mL of saturated brine and high-purity water successively, and the organic phase was dried over anhydrous sodium sulfate. Concentration, sample mixing and column chromatography gave 2.7 g of 2,6-N,N-di-bis-ferrocenylmethylaminocaproic acid (dark brown solid), with a yield of 84%.
[0114] (2) Take 0.5 g of 2,6-N,N-di-bis-ferrocenylmethylaminocaproic acid obtained in step (1), dissolve i...
Embodiment 3
[0118] This embodiment has prepared a kind of octavalent ferrocene derivative, and specific process is:
[0119] (1) Dissolve 0.5 g (983 μmol) of 6-hydroxyhexyl-2,3,4,6-tetra-O-propylamino-β-D-glucopyranoside in 25 mL of anhydrous tetrahydrofuran, and add di 1.94g (9.4mmol, 9.2eq) of ferrocene formaldehyde was stirred, and then 2.1g (9.83mmol, 10eq) of sodium triacetoxyborohydride was added to the system in batches and reacted overnight. After the reaction was detected by thin-layer chromatography (TLC), it was quenched with 30 mL of saturated sodium carbonate solution, extracted with 50 mL of ethyl acetate, then washed with 20 mL of saturated brine and high-purity water successively, and the organic phase was dried over anhydrous sodium sulfate. Concentrate, mix sample column chromatography and obtain 6-hydroxyhexyl-2,3,4,6-N,N,N,N-tetra-bisferrocene-O-propylamino-β-D-glucopyranoside ( Dark brown solid) 1.5 g, yield 73%.
[0120] (2) Take the 6-hydroxyhexyl-2,3,4,6-N,N,N,N-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com